当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
I2‐Triggered Reductive Generation of N‐Centered Iminyl Radicals: An Isatin‐to‐Quinoline Strategy for the Introduction of Primary Amides
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-02-07 , DOI: 10.1002/adsc.201701610 Qinghe Gao 1 , Zhaomin Liu 1 , Yakun Wang 1 , Xia Wu 2 , Jixia Zhang 1 , Anxin Wu 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-02-07 , DOI: 10.1002/adsc.201701610 Qinghe Gao 1 , Zhaomin Liu 1 , Yakun Wang 1 , Xia Wu 2 , Jixia Zhang 1 , Anxin Wu 2
Affiliation
|
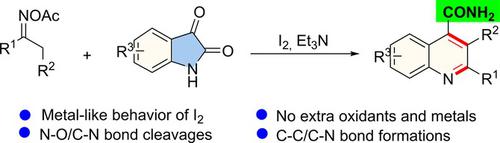
An efficient and alternative isatin‐to‐quinoline strategy illustrates the metal‐like behavior of molecular iodine in the N−O reduction of ketoxime acetates. This process involves N−O/C−N bond cleavages and C−C/C−N bond formation to furnish pharmacologically significant quinoline‐4‐carboxamide derivatives. In this process, metal catalysts and extra oxidants are unnecessary. Mechanistic studies confirm the crucial role of molecular iodine in the iminyl radical generation process, in that molecular iodine can catalyze single‐electron reduction coupling reactions in a manner similar to transition metals.
中文翻译:

I 2触发的N中心亚氨基自由基的还原生成:引入酰胺的从Isatin到Quinoline的策略
一种有效的替代品,从靛红到喹啉的策略说明了在碘化乙酸酮肟的N-O还原中分子碘的金属样行为。此过程涉及N / O / C-N键断裂和C-C / C-N键形成,以提供具有药理学意义的喹啉-4-羧酰胺衍生物。在此过程中,不需要金属催化剂和额外的氧化剂。机理研究证实了分子碘在亚氨基自由基生成过程中的关键作用,因为分子碘可以类似于过渡金属的方式催化单电子还原偶联反应。
更新日期:2018-02-07
中文翻译:

I 2触发的N中心亚氨基自由基的还原生成:引入酰胺的从Isatin到Quinoline的策略
一种有效的替代品,从靛红到喹啉的策略说明了在碘化乙酸酮肟的N-O还原中分子碘的金属样行为。此过程涉及N / O / C-N键断裂和C-C / C-N键形成,以提供具有药理学意义的喹啉-4-羧酰胺衍生物。在此过程中,不需要金属催化剂和额外的氧化剂。机理研究证实了分子碘在亚氨基自由基生成过程中的关键作用,因为分子碘可以类似于过渡金属的方式催化单电子还原偶联反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号