当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carboxyl‐Directed Conjugate Addition of C−H Bonds to α,β‐Unsaturated Ketones in Air and Water
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-02-07 , DOI: 10.1002/adsc.201701468 Wen-Jing Han 1 , Fan Pu 1 , Chao-Jun Li 2 , Zhong-Wen Liu 1 , Juan Fan 1 , Xian-Ying Shi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-02-07 , DOI: 10.1002/adsc.201701468 Wen-Jing Han 1 , Fan Pu 1 , Chao-Jun Li 2 , Zhong-Wen Liu 1 , Juan Fan 1 , Xian-Ying Shi 1
Affiliation
|
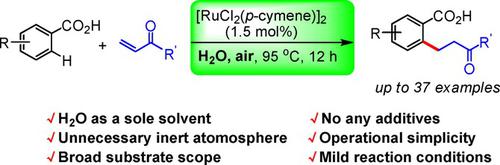
A simple ruthenium‐catalyzed conjugate addition of C−H bonds to α,β‐unsaturated ketones directed by a removable carboxyl group was developed as an effective protocol to synthesize ortho‐alkylated benzoic acids in a greener manner. Without any additives, satisfactory to excellent yields of the targeted products were achieved in neat water, and the process characterizes in mild reaction conditions (in air and water), simple operations, and broad substrate scope. Noteworthy features of this method include mild reaction conditions (in air and water), operational simplicity and broad substrate scope. The versatility and utility of the addition products were demonstrated through further transformation into commonly inaccessible but highly useful motifs of meta‐substituted alkylbenzenes and 3‐substituted isochromanones.
中文翻译:

空气和水中α-β-不饱和酮的C-H键的羧基定向共轭加成
开发了一种简单的钌催化共轭键加到可移动羧基引导的α,β-不饱和酮上的C H键,是一种以绿色方式合成邻烷基苯甲酸的有效方案。没有任何添加剂,在纯净水中就可以达到令人满意的目标产物的优异收率,而且该工艺的特点是反应条件温和(在空气和水中),操作简单且底物范围广。该方法的显着特征包括温和的反应条件(在空气和水中),操作简便和广泛的底物范围。通过进一步转化为通常难以接近但非常有用的主题图案,证明了加成产品的多功能性和实用性。间取代的烷基苯和3取代的异苯并二氢呋喃酮。
更新日期:2018-02-07
中文翻译:

空气和水中α-β-不饱和酮的C-H键的羧基定向共轭加成
开发了一种简单的钌催化共轭键加到可移动羧基引导的α,β-不饱和酮上的C H键,是一种以绿色方式合成邻烷基苯甲酸的有效方案。没有任何添加剂,在纯净水中就可以达到令人满意的目标产物的优异收率,而且该工艺的特点是反应条件温和(在空气和水中),操作简单且底物范围广。该方法的显着特征包括温和的反应条件(在空气和水中),操作简便和广泛的底物范围。通过进一步转化为通常难以接近但非常有用的主题图案,证明了加成产品的多功能性和实用性。间取代的烷基苯和3取代的异苯并二氢呋喃酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号