当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Mechanism of the Ubiquitin-Like NEDD8 Transfer in RING E3-E2∼NEDD8-Target Complex from QM/MM Free Energy Simulations
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.jcim.7b00662 Yufei Yue 1 , Yue Ma 1 , Ping Qian 2 , Hong Guo 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.jcim.7b00662 Yufei Yue 1 , Yue Ma 1 , Ping Qian 2 , Hong Guo 1
Affiliation
|
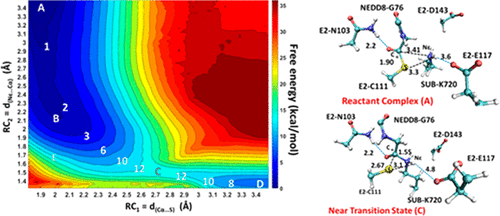
Ubiquitin-like (UBL) protein modifications play a key role in regulating protein function. In contrast to the ubiquitin (UB) and small ubiquitin-like modifier (SUMO) which are ligated to a massive segment of proteome, the UBL NEDD8 is highly selective for modifying a lysine residue on closely related cullin proteins (CULs). In this study, the X-ray structure of a trapped E3-E2∼NEDD8-target intermediate (RBX1-UBC1∼NEDD8-CUL1-DCN1) is used to build computer models, and combined quantum mechanics/molecular mechanics (QM/MM) molecular dynamics (MD) and free energy (potential of mean force) simulations are performed to investigate the catalytic mechanism of the NEDD8 transfer from E2 to the lysine residue (K720) on the substrate in the complex. The role of the active site residues is examined. The simulation results show that either E117 or D143 from E2 may be able to work as a general base catalyst to deprotonate K720 on the substrate, and K720 can then perform the nucleophilic attack on the thioester bond linking E2 and NEDD8. It is also shown that the formation of a new isopeptide bond between K720 and NEDD8 and the breaking of the thioester bond are concerted based on the computer simulations. Furthermore, the results suggest that K720 may act as a general acid catalyst to protonate the leaving group of C111 from E2. The free energy barrier for nucleophilic attack is estimated to be 14–15 kcal/mol based on the free energy simulations.
中文翻译:

QM / MM自由能模拟的环E3-E2〜NEDD8-靶复合物中泛素样NEDD8转移的催化机理
泛素样(UBL)蛋白修饰在调节蛋白功能中起关键作用。与泛素蛋白(UB)和小的泛素样修饰物(SUMO)连接到蛋白质组的很大一部分相比,UBL NEDD8对修饰紧密相关的cullin蛋白(CULs)上的赖氨酸残基具有很高的选择性。在这项研究中,使用捕获的E3-E2〜NEDD8靶中间体(RBX1-UBC1〜NEDD8-CUL1-DCN1)的X射线结构建立计算机模型,并结合了量子力学/分子力学(QM / MM)进行分子动力学(MD)和自由能(平均力势)模拟,以研究NEDD8从E2转移到复合物底物上的赖氨酸残基(K720)的催化机理。检查了活性位点残基的作用。模拟结果表明,E117的E117或D143可以作为一般的碱催化剂使底物上的K720去质子化,然后K720可以对连接E2和NEDD8的硫酯键进行亲核攻击。还表明,基于计算机模拟,在K720和NEDD8之间形成新的异肽键和硫酯键的断裂是一致的。此外,结果表明,K720可以充当一般的酸催化剂,以质子化E2中C111的离去基团。根据自由能模拟,亲核攻击的自由能垒估计为14-15 kcal / mol。还表明,基于计算机模拟,在K720和NEDD8之间形成新的异肽键和硫酯键的断裂是一致的。此外,结果表明,K720可以充当一般的酸催化剂,以质子化E2中C111的离去基团。根据自由能模拟,亲核攻击的自由能垒估计为14-15 kcal / mol。还表明,基于计算机模拟,在K720和NEDD8之间形成新的异肽键和硫酯键的断裂是一致的。此外,结果表明,K720可以充当一般的酸催化剂,以质子化E2中C111的离去基团。根据自由能模拟,亲核攻击的自由能垒估计为14-15 kcal / mol。
更新日期:2018-02-05
中文翻译:

QM / MM自由能模拟的环E3-E2〜NEDD8-靶复合物中泛素样NEDD8转移的催化机理
泛素样(UBL)蛋白修饰在调节蛋白功能中起关键作用。与泛素蛋白(UB)和小的泛素样修饰物(SUMO)连接到蛋白质组的很大一部分相比,UBL NEDD8对修饰紧密相关的cullin蛋白(CULs)上的赖氨酸残基具有很高的选择性。在这项研究中,使用捕获的E3-E2〜NEDD8靶中间体(RBX1-UBC1〜NEDD8-CUL1-DCN1)的X射线结构建立计算机模型,并结合了量子力学/分子力学(QM / MM)进行分子动力学(MD)和自由能(平均力势)模拟,以研究NEDD8从E2转移到复合物底物上的赖氨酸残基(K720)的催化机理。检查了活性位点残基的作用。模拟结果表明,E117的E117或D143可以作为一般的碱催化剂使底物上的K720去质子化,然后K720可以对连接E2和NEDD8的硫酯键进行亲核攻击。还表明,基于计算机模拟,在K720和NEDD8之间形成新的异肽键和硫酯键的断裂是一致的。此外,结果表明,K720可以充当一般的酸催化剂,以质子化E2中C111的离去基团。根据自由能模拟,亲核攻击的自由能垒估计为14-15 kcal / mol。还表明,基于计算机模拟,在K720和NEDD8之间形成新的异肽键和硫酯键的断裂是一致的。此外,结果表明,K720可以充当一般的酸催化剂,以质子化E2中C111的离去基团。根据自由能模拟,亲核攻击的自由能垒估计为14-15 kcal / mol。还表明,基于计算机模拟,在K720和NEDD8之间形成新的异肽键和硫酯键的断裂是一致的。此外,结果表明,K720可以充当一般的酸催化剂,以质子化E2中C111的离去基团。根据自由能模拟,亲核攻击的自由能垒估计为14-15 kcal / mol。



























 京公网安备 11010802027423号
京公网安备 11010802027423号