当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling the Interaction of Molecular Iodine with MAPbI3: A Probe of Lead-Halide Perovskites Defect Chemistry
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acsenergylett.7b01244 Daniele Meggiolaro 1, 2 , Edoardo Mosconi 1, 3 , Filippo De Angelis 1, 2, 3
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acsenergylett.7b01244 Daniele Meggiolaro 1, 2 , Edoardo Mosconi 1, 3 , Filippo De Angelis 1, 2, 3
Affiliation
|
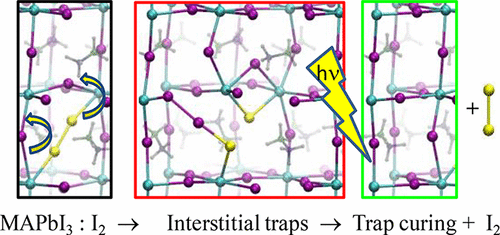
Understanding the defect chemistry of lead-halide perovskites is of paramount importance to further progress toward exploitation of these materials. Here, we combine recent experimental observations on the behavior of MAPbI3 upon exposure to I2 vapor with first-principles calculations to extract a global picture of defect chemistry in lead-halide perovskites. By matching the reported experimental observables we disclose the origin of the p-doping observed upon exposing MAPbI3 to I2 and highlight its consequences on the charge and ion transport and trapping activity. Electron/hole traps related to positive/negative interstitial iodine dominate the defect chemistry in intrinsic conditions, while in p-doped MAPbI3, electrons are mainly trapped by positive interstitial iodine and neutral lead vacancies. I2 spontaneously dissociates on iodine vacancies, leading to vacancy passivation and to the formation of positive interstitial iodine. I2 spontaneously dissociates on nondefective MAPbI3(001) surfaces to form pairs of negative/positive interstitial iodine. Upon trapping a hole/electron pair at negative/positive interstitial iodine, I2 release becomes thermodynamically favored, possibly representing a photoinduced trap-curing mechanism.
中文翻译:

分子碘与MAPbI 3相互作用的建模:卤化钙钛矿铅缺陷化学的探索
了解卤化钙钛矿的缺陷化学对于进一步开发这些材料至关重要。在这里,我们将最近对MAPbI 3暴露于I 2蒸气后的行为的实验观察结果与第一性原理计算相结合,以提取卤化钙钛矿中缺陷化学的全局图。通过匹配报道的实验可观察物,我们公开了将MAPbI 3暴露于I 2时观察到的p掺杂的起源,并突出了其对电荷,离子迁移和俘获活性的影响。在固有条件下,与正/负间隙碘有关的电子/空穴陷阱占主导地位,而在p掺杂的MAPbI 3中,电子/空穴陷阱占主导地位。,电子主要被正性间隙碘和中性铅空位俘获。I 2自发地解离碘空位,导致空位钝化并形成正性间隙碘。I 2在无缺陷的MAPbI 3(001)表面上自发解离,形成成对的负/正质间隙碘。在将空穴/电子对俘获在负/正间隙碘上时,I 2的释放在热力学上变得有利,可能代表了光诱导的俘获固化机制。
更新日期:2018-01-25
中文翻译:

分子碘与MAPbI 3相互作用的建模:卤化钙钛矿铅缺陷化学的探索
了解卤化钙钛矿的缺陷化学对于进一步开发这些材料至关重要。在这里,我们将最近对MAPbI 3暴露于I 2蒸气后的行为的实验观察结果与第一性原理计算相结合,以提取卤化钙钛矿中缺陷化学的全局图。通过匹配报道的实验可观察物,我们公开了将MAPbI 3暴露于I 2时观察到的p掺杂的起源,并突出了其对电荷,离子迁移和俘获活性的影响。在固有条件下,与正/负间隙碘有关的电子/空穴陷阱占主导地位,而在p掺杂的MAPbI 3中,电子/空穴陷阱占主导地位。,电子主要被正性间隙碘和中性铅空位俘获。I 2自发地解离碘空位,导致空位钝化并形成正性间隙碘。I 2在无缺陷的MAPbI 3(001)表面上自发解离,形成成对的负/正质间隙碘。在将空穴/电子对俘获在负/正间隙碘上时,I 2的释放在热力学上变得有利,可能代表了光诱导的俘获固化机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号