当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Matched Coupling of Propargylic Carbonates with Cyclopropanols
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b03637 Penglin Wu 1 , Minqiang Jia 1 , Weilong Lin 2 , Shengming Ma 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b03637 Penglin Wu 1 , Minqiang Jia 1 , Weilong Lin 2 , Shengming Ma 1, 2
Affiliation
|
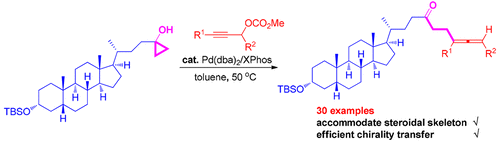
The ring opening–coupling reaction of cyclopropanols with propargylic carbonates affording synthetically attractive allenyl ketones has been developed. The mechanism involves the ligand-exchange reaction of in situ formed allenyl palladium methoxide with cyclopropanols followed by carbon–carbon bond cleavage and reductive elimination. The reactions proceeded smoothly under mild reaction conditions with Pd(0)/XPhos catalysis in the absence of any external base and displayed a wide scope and application to a steroidal skeleton. The efficiency of chirality transfer and synthetic utility of the allene products have also been demonstrated.
中文翻译:

炔丙基碳酸酯与环丙醇的匹配偶联
已经开发了环丙醇与碳酸丙炔酯的开环偶联反应,提供了合成上有吸引力的烯丙基酮。该机理涉及原位形成的烯丙基甲醇钯与环丙醇的配体交换反应,然后进行碳-碳键裂解和还原消除。在没有任何外部碱的情况下,在Pd(0)/ XPhos催化下,在温和的反应条件下,反应可以顺利进行,并显示出广泛的适用范围和对甾体骨架的应用。还已经证明了丙二烯产物的手性转移效率和合成效用。
更新日期:2018-01-18
中文翻译:

炔丙基碳酸酯与环丙醇的匹配偶联
已经开发了环丙醇与碳酸丙炔酯的开环偶联反应,提供了合成上有吸引力的烯丙基酮。该机理涉及原位形成的烯丙基甲醇钯与环丙醇的配体交换反应,然后进行碳-碳键裂解和还原消除。在没有任何外部碱的情况下,在Pd(0)/ XPhos催化下,在温和的反应条件下,反应可以顺利进行,并显示出广泛的适用范围和对甾体骨架的应用。还已经证明了丙二烯产物的手性转移效率和合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号