当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remarkable similarity in Plasmodium falciparum and Plasmodium vivax geranylgeranyl diphosphate synthase dynamics and its implication for antimalarial drug design
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-02-04 , DOI: 10.1111/cbdd.13170 Aishwarya Venkatramani 1, 2, 3 , Clarisse Gravina Ricci 1, 2, 3 , Eric Oldfield 4 , J Andrew McCammon 1, 2, 3
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-02-04 , DOI: 10.1111/cbdd.13170 Aishwarya Venkatramani 1, 2, 3 , Clarisse Gravina Ricci 1, 2, 3 , Eric Oldfield 4 , J Andrew McCammon 1, 2, 3
Affiliation
|
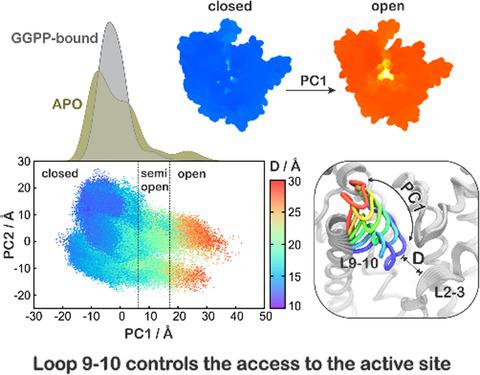
Malaria, mainly caused by Plasmodium falciparum and Plasmodium vivax, has been a growing cause of morbidity and mortality. P. falciparum is more lethal than is P. vivax, but there is a vital need for effective drugs against both species. Geranylgeranyl diphosphate synthase (GGPPS) is an enzyme involved in the biosynthesis of quinones and in protein prenylation and has been proposed to be a malaria drug target. However, the structure of P. falciparumGGPPS (PfGGPPS) has not been determined, due to difficulties in crystallization. Here, we created a PfGGPPS model using the homologous P.vivaxGGPPS X‐ray structure as a template. We simulated the modeled PfGGPPS as well as PvGGPPS using conventional and Gaussian accelerated molecular dynamics in both apo‐ and GGPP‐bound states. The MD simulations revealed a striking similarity in the dynamics of both enzymes with loop 9‐10 controlling access to the active site. We also found that GGPP stabilizes PfGGPPS and PvGGPPS into closed conformations and via similar mechanisms. Shape‐based analysis of the binding sites throughout the simulations suggests that the two enzymes will be readily targeted by the same inhibitors. Finally, we produced three MD‐validated conformations of PfGGPPS to be used in future virtual screenings for potential new antimalarial drugs acting on both PvGGPPS and PfGGPPS.
中文翻译:

恶性疟原虫和间日疟原虫香叶基香叶基二磷酸合酶动力学的显着相似性及其对抗疟药物设计的意义
疟疾主要由恶性疟原虫和间日疟原虫引起,已成为发病率和死亡率日益增长的原因。恶性疟原虫比间日疟原虫更具致死性,但迫切需要针对这两种物种的有效药物。香叶基香叶基二磷酸合酶 (GGPPS) 是一种参与醌生物合成和蛋白质异戊二烯化的酶,已被提议作为疟疾药物靶点。然而,由于结晶困难,恶性疟原虫GGPPS( Pf GGPPS)的结构尚未确定。在这里,我们使用同源P.vivax GGPPS X 射线结构作为模板创建了Pf GGPPS 模型。我们在apo和 GGPP 结合态下使用传统和高斯加速分子动力学模拟了建模的Pf GGPPS 和Pv GGPPS。 MD 模拟揭示了两种酶的动力学具有惊人的相似性,其中环 9-10 控制着对活性位点的访问。我们还发现 GGPP通过类似的机制将Pf GGPPS 和Pv GGPPS 稳定为闭合构象。在整个模拟过程中对结合位点进行的基于形状的分析表明,这两种酶很容易被相同的抑制剂靶向。最后,我们生产了三种 MD 验证的Pf GGPPS 构象,用于未来虚拟筛选同时作用于Pv GGPPS 和Pf GGPPS 的潜在新型抗疟药物。
更新日期:2018-02-04
中文翻译:

恶性疟原虫和间日疟原虫香叶基香叶基二磷酸合酶动力学的显着相似性及其对抗疟药物设计的意义
疟疾主要由恶性疟原虫和间日疟原虫引起,已成为发病率和死亡率日益增长的原因。恶性疟原虫比间日疟原虫更具致死性,但迫切需要针对这两种物种的有效药物。香叶基香叶基二磷酸合酶 (GGPPS) 是一种参与醌生物合成和蛋白质异戊二烯化的酶,已被提议作为疟疾药物靶点。然而,由于结晶困难,恶性疟原虫GGPPS( Pf GGPPS)的结构尚未确定。在这里,我们使用同源P.vivax GGPPS X 射线结构作为模板创建了Pf GGPPS 模型。我们在apo和 GGPP 结合态下使用传统和高斯加速分子动力学模拟了建模的Pf GGPPS 和Pv GGPPS。 MD 模拟揭示了两种酶的动力学具有惊人的相似性,其中环 9-10 控制着对活性位点的访问。我们还发现 GGPP通过类似的机制将Pf GGPPS 和Pv GGPPS 稳定为闭合构象。在整个模拟过程中对结合位点进行的基于形状的分析表明,这两种酶很容易被相同的抑制剂靶向。最后,我们生产了三种 MD 验证的Pf GGPPS 构象,用于未来虚拟筛选同时作用于Pv GGPPS 和Pf GGPPS 的潜在新型抗疟药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号