当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytically Enantioselective Synthesis of Acyclic α-Tertiary Amines through Desymmetrization of 2-Substituted 2-Nitro-1,3-diols
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b03581 Shan-Shui Meng 1 , Wu-Bang Tang 1 , Wen-Hua Zheng 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b03581 Shan-Shui Meng 1 , Wu-Bang Tang 1 , Wen-Hua Zheng 1
Affiliation
|
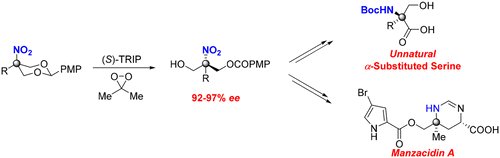
Highly enantioselective synthesis of acyclic α-tertiary amines through asymmetric desymmetrization is reported. This approach is based on chiral phosphoric acid mediated, enantioselective, oxidative desymmetrization of 2-substituted 2-nitro-1,3-diolbenzylidine acetals in the presence of DMDO as an oxidant. The method allows for the formation of a wide variety of chiral 2-nitro-1,3-diols in high enantioselectivity, which could be transformed into optically pure, unnatural α-alkyl series. The synthetic utility of this method has been further demonstrated by the expedient construction of the core structure of natural products manzacidins enantioselectively.
中文翻译:

通过2-取代的2-硝基-1,3-二醇的不对称化催化无环α-叔胺的催化合成
据报道,通过不对称脱对称性,对映体可以高度对映选择性地合成无环α-叔胺。该方法基于在DMDO作为氧化剂存在下2-取代的2-硝基-1,3-二醇苄基乙缩醛的手性磷酸介导的对映体选择性氧化氧化脱对称。该方法允许以高对映选择性形成多种手性2-硝基-1,3-二醇,其可以转化为光学纯的,非天然的α-烷基系列。该方法的合成效用已通过对映体选择性地天然构建花青素的核心结构得到了进一步证明。
更新日期:2018-01-18
中文翻译:

通过2-取代的2-硝基-1,3-二醇的不对称化催化无环α-叔胺的催化合成
据报道,通过不对称脱对称性,对映体可以高度对映选择性地合成无环α-叔胺。该方法基于在DMDO作为氧化剂存在下2-取代的2-硝基-1,3-二醇苄基乙缩醛的手性磷酸介导的对映体选择性氧化氧化脱对称。该方法允许以高对映选择性形成多种手性2-硝基-1,3-二醇,其可以转化为光学纯的,非天然的α-烷基系列。该方法的合成效用已通过对映体选择性地天然构建花青素的核心结构得到了进一步证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号