当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
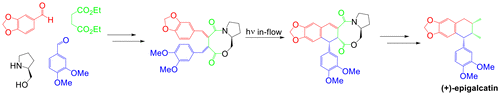
Flow Photochemistry as a Tool for the Total Synthesis of (+)-Epigalcatin
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b03974 Kamil Lisiecki 1 , Zbigniew Czarnocki 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b03974 Kamil Lisiecki 1 , Zbigniew Czarnocki 1
Affiliation
|
The first total synthesis of (+)-epigalcatin was completed in a highly stereoselective manner starting from piperonal, 3,4-dimethylbenzaldehyde, and diethyl succinate. l-Prolinol was used as a chiral auxiliary. The crucial step in this procedure involves the construction of the cyclolignan framework by continuous-flow photocyclization of a chiral atropisomeric 1,2-bisbenzylidenesuccinate amide ester.
中文翻译:

流动光化学作为(+)-表甜抗素全合成的工具
(+)-表皮角质素的第一个全合成是从胡椒醛,3,4-二甲基苯甲醛和琥珀酸二乙酯以高度立体选择性的方式完成的。1-脯氨醇用作手性助剂。该方法中的关键步骤涉及通过手性阻转异构体1,2-双亚苄基琥珀酸酰胺酯的连续流动光环化来构建环木聚糖骨架。
更新日期:2018-01-18
中文翻译:

流动光化学作为(+)-表甜抗素全合成的工具
(+)-表皮角质素的第一个全合成是从胡椒醛,3,4-二甲基苯甲醛和琥珀酸二乙酯以高度立体选择性的方式完成的。1-脯氨醇用作手性助剂。该方法中的关键步骤涉及通过手性阻转异构体1,2-双亚苄基琥珀酸酰胺酯的连续流动光环化来构建环木聚糖骨架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号