当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integration of Open Metal Sites and Lewis Basic Sites for Construction of a Cu MOF with a Rare Chiral Oh‐type cage for high performance in methane purification
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-02-19 , DOI: 10.1002/chem.201800010 Lingkun Meng 1 , Ziyuan Niu 1 , Chen Liang 1 , Xinglong Dong 2 , Kang Liu 3 , Guanghua Li 1 , Chunguang Li 1 , Yu Han 2 , Zhan Shi 1 , Shouhua Feng 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-02-19 , DOI: 10.1002/chem.201800010 Lingkun Meng 1 , Ziyuan Niu 1 , Chen Liang 1 , Xinglong Dong 2 , Kang Liu 3 , Guanghua Li 1 , Chunguang Li 1 , Yu Han 2 , Zhan Shi 1 , Shouhua Feng 1
Affiliation
|
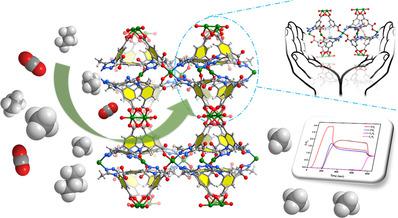
A Cu metal‐organic framework (MOF), [Cu4(PMTD)2(H2O)3]⋅20 H2O, 1, (where PMTD is 1,4‐phenylenebis(5‐methyl‐4H‐1,2,4‐triazole‐3,4‐diyl)bis(5‐carboxylato‐3,1‐phenylene)bis(hydroperoxymethanide)), with a rare chiral Oh‐type cage, and dual functionalities of open metal sites and Lewis basic sites, based on a designed U‐shaped ligand, was synthesized by hydrothermal methods. It exhibits high CO2, C2, and C3 hydrocarbon storage capacity under atmospheric pressure, as well as high H2 (1.96 wt.%) adsorption capacity at 77 K. Methane purification capacity was tested and verified step by step. Isosteric heats (Qst) studies reveal that CH4 has the weakest van der Waals host–guest interactions among the seven gases at 298 K. Ideal adsorbed solution theory (IAST) calculation reveals that compound 1 is more selective toward CO2, C2H6, and C3H8 over CH4 in further calculating its separation capacity, as exemplified for CO2/CH4 (50:50, 5:95), C2H6/CH4 (50:50, 5:95), or C3H8/CH4 (50:50, 5:95) binary gas mixtures. Breakthrough experiments show that 1 has a significantly higher adsorption capacity for CO2, C2H6, and C3H8 than CH4. The selective adsorption properties of 1 make it a promising candidate for methane purification.
中文翻译:

集成开放式金属场地和Lewis基础场地,以构建具有稀有手性Oh型笼的Cu MOF,以实现甲烷净化的高性能
铜金属有机骨架(MOF),[Cu 4(PMTD)2(H 2 O)3 ] ⋅20 H 2 O,1(其中PMTD为1,4-亚苯基二(5-甲基-4 H -1) ,2,4-三唑-3,4-二基)双(5-羧基-3,1-亚苯基)双(氢过氧甲烷),具有罕见的手性O h型笼,并且具有开放金属位点和Lewis的双重功能通过水热法合成了基于设计的U型配体的基本位点。它在大气压下显示出高的CO 2,C 2和C 3碳氢化合物存储能力,以及高的H 2。(1.96 wt。%)的77 K吸附容量。逐步测试并验证了甲烷的纯化能力。等容热(Q st)研究表明,在298 K时,CH 4的七个气体之间的范德华主体与客体之间的相互作用最弱。理想吸附溶液理论(IAST)计算表明,化合物1对CO 2,C 2的选择性更高H 6和C 3 H 8在CH 4上的分离能力,以进一步计算其分离能力,例如对CO 2 / CH 4(50:50,5:95),C 2 H 6 / CH 4(50:50,5:95)或C 3 H 8 / CH 4(50:50,5:95)二元气体混合物。突破性实验表明,1具有比CH 4更高的对CO 2,C 2 H 6和C 3 H 8的吸附能力。的选择性吸附性能1使其成为甲烷纯化有希望的候选。
更新日期:2018-02-19
中文翻译:

集成开放式金属场地和Lewis基础场地,以构建具有稀有手性Oh型笼的Cu MOF,以实现甲烷净化的高性能
铜金属有机骨架(MOF),[Cu 4(PMTD)2(H 2 O)3 ] ⋅20 H 2 O,1(其中PMTD为1,4-亚苯基二(5-甲基-4 H -1) ,2,4-三唑-3,4-二基)双(5-羧基-3,1-亚苯基)双(氢过氧甲烷),具有罕见的手性O h型笼,并且具有开放金属位点和Lewis的双重功能通过水热法合成了基于设计的U型配体的基本位点。它在大气压下显示出高的CO 2,C 2和C 3碳氢化合物存储能力,以及高的H 2。(1.96 wt。%)的77 K吸附容量。逐步测试并验证了甲烷的纯化能力。等容热(Q st)研究表明,在298 K时,CH 4的七个气体之间的范德华主体与客体之间的相互作用最弱。理想吸附溶液理论(IAST)计算表明,化合物1对CO 2,C 2的选择性更高H 6和C 3 H 8在CH 4上的分离能力,以进一步计算其分离能力,例如对CO 2 / CH 4(50:50,5:95),C 2 H 6 / CH 4(50:50,5:95)或C 3 H 8 / CH 4(50:50,5:95)二元气体混合物。突破性实验表明,1具有比CH 4更高的对CO 2,C 2 H 6和C 3 H 8的吸附能力。的选择性吸附性能1使其成为甲烷纯化有希望的候选。











































 京公网安备 11010802027423号
京公网安备 11010802027423号