当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ternary HNO3–H2SO4–H2O Mixtures: A Simplified Approach for the Calculation of the Equilibrium Composition
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-01-27 00:00:00 , DOI: 10.1021/acs.iecr.7b04193 Danilo Russo 1 , Raffaele Marotta 1 , Mario Commodo 2 , Roberto Andreozzi 1 , Ilaria Di Somma 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-01-27 00:00:00 , DOI: 10.1021/acs.iecr.7b04193 Danilo Russo 1 , Raffaele Marotta 1 , Mario Commodo 2 , Roberto Andreozzi 1 , Ilaria Di Somma 2
Affiliation
|
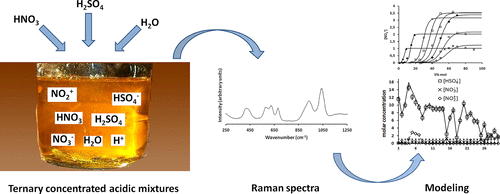
Concentrated ternary mixture of nitric acid, sulfuric acid, and water are often used in the chemical industry for a wide range of applications. Among these, nitrations of organic substrates are the most common. Nevertheless, there is still a poor understanding of speciation of the acids in the mixtures, and different simplified or semiempirical approaches have been found in the literature to model nitration kinetics by mixed acids. Most of the found investigations are relevant in restricted ranges of experimental conditions, resulting in a fragmentary vision. In this work, most of the approaches found in the literature were reviewed in order to define their limits of validity, and new empirical equations for the estimation of nitronium ion concentration are proposed. Moreover, on the basis of original experimental data, a simplified model is proposed to calculate the dissociation of acids at varying experimental conditions, and the main assumptions reported in the literature were verified. Adopted simplifying assumptions were verified at sulfuric acid concentrations higher than 2.1 mol·L–1 and nitric acid concentrations ranging from 2.0 to 11 mol·L–1.
中文翻译:

三元HNO 3 -H 2 SO 4 -H 2 O混合物:平衡组成计算的简化方法
硝酸,硫酸和水的浓三元混合物通常在化学工业中用于广泛的应用。其中,有机底物的硝化是最常见的。然而,对于混合物中酸的形态仍知之甚少,并且在文献中发现了不同的简化或半经验方法来模拟混合酸的硝化动力学。大部分发现的研究都与有限的实验条件有关,导致视力不完整。在这项工作中,对文献中发现的大多数方法进行了综述,以定义其有效性极限,并提出了用于估算硝态氮离子浓度的新的经验方程式。而且,根据原始实验数据,提出了一个简化的模型来计算在不同实验条件下酸的离解,并验证了文献中报道的主要假设。在硫酸浓度高于2.1mol·L的情况下,采用的简化假设得到了验证–1和硝酸浓度范围从2.0到11mol·L –1。
更新日期:2018-01-27
中文翻译:

三元HNO 3 -H 2 SO 4 -H 2 O混合物:平衡组成计算的简化方法
硝酸,硫酸和水的浓三元混合物通常在化学工业中用于广泛的应用。其中,有机底物的硝化是最常见的。然而,对于混合物中酸的形态仍知之甚少,并且在文献中发现了不同的简化或半经验方法来模拟混合酸的硝化动力学。大部分发现的研究都与有限的实验条件有关,导致视力不完整。在这项工作中,对文献中发现的大多数方法进行了综述,以定义其有效性极限,并提出了用于估算硝态氮离子浓度的新的经验方程式。而且,根据原始实验数据,提出了一个简化的模型来计算在不同实验条件下酸的离解,并验证了文献中报道的主要假设。在硫酸浓度高于2.1mol·L的情况下,采用的简化假设得到了验证–1和硝酸浓度范围从2.0到11mol·L –1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号