当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron and Arsenic Speciation During As(III) Oxidation by Manganese Oxides in the Presence of Fe(II): Molecular-Level Characterization Using XAFS, Mössbauer, and TEM Analysis
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00119 Yun Wu 1 , Ravi K. Kukkadapu 2 , Kenneth J. T. Livi 3 , Wenqian Xu 4 , Wei Li 1, 5 , Donald L. Sparks 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00119 Yun Wu 1 , Ravi K. Kukkadapu 2 , Kenneth J. T. Livi 3 , Wenqian Xu 4 , Wei Li 1, 5 , Donald L. Sparks 1
Affiliation
|
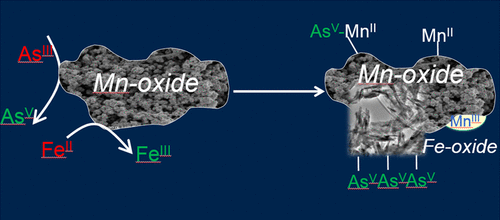
The redox state and speciation of the metalloid arsenic (As) determine its toxicity and mobility. Knowledge of biogeochemical processes influencing the As redox state is therefore important to understand and predict its environmental behavior. Many previous studies examined As(III) oxidation by various Mn-oxides, but little is known concerning environmental influences (e.g., coexisting ions) on the process. In this study, we investigated the mechanisms of As(III) oxidation by a poorly crystalline hexagonal birnessite (δ-MnO2) in the presence of dissolved Fe(II) using X-ray absorption spectroscopy, Mössbauer spectroscopy, and transmission electron microscopy (TEM) coupled with energy-dispersive X-ray spectroscopy (EDS). The As K-edge X-ray absorption near edge spectroscopy (XANES) analysis revealed that, at low Fe(II) concentration (100 μM), As(V) was the predominant As species on the solid phase, whereas at higher Fe(II) concentrations (200–1000 μM), both As(III) and As(V) were sorbed on the solid phase. As K-edge extended X-ray absorption fine structure spectroscopy (EXAFS) analysis showed an increasing As–Mn/Fe distance over time, indicating As prefers to bind with the newly formed Fe(III)-(hydr)oxides. Both As(III) and (V) adsorbed on Fe(III)-(hydr)oxides as a bidentate binuclear corner-sharing complex. Both Mössbauer and TEM-EDS investigations demonstrated that oxidized Fe(III) products formed during Fe(II) oxidation by δ-MnO2 were predominantly ferrihydrite-, goethite-, and ferric arsenate-like compounds. However, Fe EXAFS analysis also suggested the formation of a small amount of lepidocrocite. The Mn K-edge XANES data indicated that As(III) oxidation occurs as a two electron transfer with δ-MnO2 and that the observed Mn(III) is due to conproportionation of surface-sorbed Mn(II) with Mn(IV) in the δ-MnO2 structure. This study reveals that the mechanisms of As(III) oxidation by δ-MnO2 in the presence of Fe(II) are very complex, involving many simultaneous reactions, and the formation of Fe(III)-(hydr)oxides plays a very important role in reducing As mobility.
中文翻译:

Fe(II)存在下锰氧化物氧化As(III)时的铁和砷形态:使用XAFS,Mössbauer和TEM分析进行分子水平表征
准金属砷(As)的氧化还原状态和形态决定了它的毒性和迁移率。因此,了解影响As氧化还原状态的生物地球化学过程的知识对于理解和预测其环境行为很重要。先前的许多研究都研究了各种锰氧化物对As(III)的氧化作用,但是关于环境对工艺的影响(例如,共存离子)知之甚少。在这项研究中,我们通过一个低结晶六边形水钠锰矿(δ-MnO的研究作为(III)氧化的机制2)在溶解的Fe(II)存在下使用X射线吸收光谱,Mössbauer光谱和透射电子显微镜(TEM)结合能量色散X射线光谱(EDS)。As K边缘X射线吸收近缘光谱(XANES)分析表明,在低Fe(II)浓度(100μM)下,As(V)是固相中的主要As物种,而在Fe(II)较高时II)浓度(200–1000μM),As(III)和As(V)均吸附在固相上。随着K边缘扩展X射线吸收精细结构光谱(EXAFS)分析显示,随着时间的推移,As-Mn / Fe距离增加,这表明As倾向于与新形成的Fe(III)-(hydr)oxides结合。As(III)和(V)都以双齿双核角共享络合物的形式吸附在Fe(III)-(氢)氧化物上。其中2种主要为亚铁酸盐,针铁矿和砷酸铁样化合物。但是,Fe EXAFS分析还表明形成了少量的纤铁矿。Mn的K边缘XANES数据表明,如(III)发生氧化,与δ-MnO的两个电子转移2和所观察到的锰(III)是由于conproportionation表面吸附的Mn(II)与锰(IV)在δ-的MnO 2结构。这项研究表明,作为(III)氧化的由δ-MnO的机制2中的Fe(II)的存在是非常复杂的,涉及到许多同时反应,和Fe(III)的形成- (氢)氧化物起着非常在减少As流动性中起重要作用。
更新日期:2018-01-17
中文翻译:

Fe(II)存在下锰氧化物氧化As(III)时的铁和砷形态:使用XAFS,Mössbauer和TEM分析进行分子水平表征
准金属砷(As)的氧化还原状态和形态决定了它的毒性和迁移率。因此,了解影响As氧化还原状态的生物地球化学过程的知识对于理解和预测其环境行为很重要。先前的许多研究都研究了各种锰氧化物对As(III)的氧化作用,但是关于环境对工艺的影响(例如,共存离子)知之甚少。在这项研究中,我们通过一个低结晶六边形水钠锰矿(δ-MnO的研究作为(III)氧化的机制2)在溶解的Fe(II)存在下使用X射线吸收光谱,Mössbauer光谱和透射电子显微镜(TEM)结合能量色散X射线光谱(EDS)。As K边缘X射线吸收近缘光谱(XANES)分析表明,在低Fe(II)浓度(100μM)下,As(V)是固相中的主要As物种,而在Fe(II)较高时II)浓度(200–1000μM),As(III)和As(V)均吸附在固相上。随着K边缘扩展X射线吸收精细结构光谱(EXAFS)分析显示,随着时间的推移,As-Mn / Fe距离增加,这表明As倾向于与新形成的Fe(III)-(hydr)oxides结合。As(III)和(V)都以双齿双核角共享络合物的形式吸附在Fe(III)-(氢)氧化物上。其中2种主要为亚铁酸盐,针铁矿和砷酸铁样化合物。但是,Fe EXAFS分析还表明形成了少量的纤铁矿。Mn的K边缘XANES数据表明,如(III)发生氧化,与δ-MnO的两个电子转移2和所观察到的锰(III)是由于conproportionation表面吸附的Mn(II)与锰(IV)在δ-的MnO 2结构。这项研究表明,作为(III)氧化的由δ-MnO的机制2中的Fe(II)的存在是非常复杂的,涉及到许多同时反应,和Fe(III)的形成- (氢)氧化物起着非常在减少As流动性中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号