当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Total Syntheses of Callistrilones A, B, and D
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03815 Dattatraya H. Dethe 1 , Balu D. Dherange 1 , Saikat Das 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03815 Dattatraya H. Dethe 1 , Balu D. Dherange 1 , Saikat Das 1
Affiliation
|
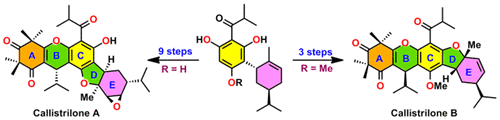
A biomimetic total syntheses of antibacterial natural products (±)-callistrilones A, B, and D, the first triketone–phloroglucinol–monoterpene hybrids with an unprecedented [1]benzofuro[2,3-a]xanthene and [1]benzofuro[3,2-b]xanthene pentacyclic ring system along with the postulated biosynthetic intermediate, isolated from the leaves of Callistemon rigidus, were achieved. The total synthesis features highly regio- and diastereoselective catalytic Friedel–Crafts alkylation, palladium-catalyzed Wacker-type oxidative cyclization, Michael addition, and late-stage diastereoselective epoxide formation from the extremely hindered β face as key steps.
中文翻译:

仿生总合成Callistrilones A,B和D
仿生的抗菌天然产物(±)-卡斯地酮A,B和D的仿生全合成,这是第一个三酮-间苯三酚-单萜杂合物,具有前所未有的[1]苯并呋喃[2,3- a ]氧杂蒽和[1]苯并呋喃[3]分离自硬叶Callistemon硬叶的,2- b ]并氧杂蒽五环系统以及假定的生物合成中间体。整个合成过程具有高度区域选择性和非对映选择性催化Friedel-Crafts烷基化,钯催化的Wacker型氧化环化,迈克尔加成以及从极度受阻的β面形成后期非对映选择性环氧化物的关键步骤。
更新日期:2018-01-17
中文翻译:

仿生总合成Callistrilones A,B和D
仿生的抗菌天然产物(±)-卡斯地酮A,B和D的仿生全合成,这是第一个三酮-间苯三酚-单萜杂合物,具有前所未有的[1]苯并呋喃[2,3- a ]氧杂蒽和[1]苯并呋喃[3]分离自硬叶Callistemon硬叶的,2- b ]并氧杂蒽五环系统以及假定的生物合成中间体。整个合成过程具有高度区域选择性和非对映选择性催化Friedel-Crafts烷基化,钯催化的Wacker型氧化环化,迈克尔加成以及从极度受阻的β面形成后期非对映选择性环氧化物的关键步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号