当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization of NaBH4 in Methanol Using a Catalytic Amount of NaOMe. Reduction of Esters and Lactones at Room Temperature without Solvent-Induced Loss of Hydride
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.joc.7b02993 Prasanth C. P. 1 , Ebbin Joseph 1 , Abhijith A 1 , Nair D. S. 1 , Ibrahim Ibnusaud 1 , Jevgenij Raskatov 2 , Bakthan Singaram 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.joc.7b02993 Prasanth C. P. 1 , Ebbin Joseph 1 , Abhijith A 1 , Nair D. S. 1 , Ibrahim Ibnusaud 1 , Jevgenij Raskatov 2 , Bakthan Singaram 2
Affiliation
|
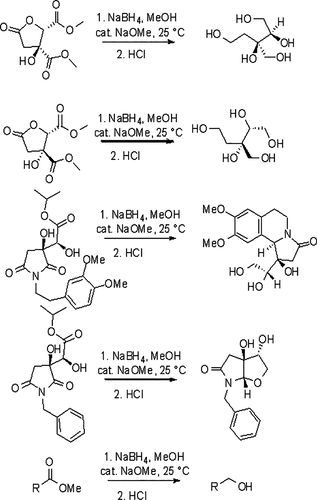
Rapid reaction of NaBH4 with MeOH precludes its use as a solvent for large-scale ester reductions. We have now learned that a catalytic amount of NaOMe (5 mol %) stabilizes NaBH4 solutions in methanol at 25 °C and permits the use of these solutions for the reduction of esters to alcohols. The generality of this reduction method was demonstrated using 22 esters including esters of naturally occurring chiral γ-butyrolactone containing dicarboxylic acids. This method permits the chemoselective reductions of esters in the presence of cyano and nitro groups and the reductive cyclization of a pyrrolidinedione ester to a fused five-membered furo[2,3-b]pyrrole and a (−)-crispine A analogue in high optical and chemical yields. Lactones, aliphatic esters, aromatic esters containing electron-withdrawing groups, and heteroaryl esters are reduced more rapidly than aryl esters containing electron-donating groups. The 11B NMR spectrum of the NaOMe-stabilized NaBH4 solutions showed a minor quartet due to monomethoxyborohydride (NaBH3OMe) that persisted up to 18 h at 25 °C. We postulate that NaBH3OMe is probably the active reducing agent. In support of this hypothesis, the activation barrier for hydride transfer from BH3(OMe)− onto benzoic acid methyl ester was calculated as 18.3 kcal/mol.
中文翻译:

使用催化量的NaOMe稳定甲醇中的NaBH 4。在室温下减少酯和内酯,而不会导致溶剂引起的氢化物损失
NaBH 4与MeOH的快速反应排除了其作为溶剂进行大规模酯还原的用途。我们现已了解到,催化量的NaOMe(5摩尔%)可使NaBH 4溶液在25°C的甲醇中稳定,并允许将这些溶液用于将酯还原为醇类。使用包括天然存在的含手性γ-丁内酯的二羧酸的酯在内的22种酯证明了该还原方法的普遍性。该方法允许在氰基和硝基存在下酯的化学选择性还原,以及吡咯烷二酮酯的还原环化成稠合的五元呋喃[2,3- b]。]吡咯和(-)-crispine A类似物,具有较高的光学和化学收率。内酯,脂族酯,含有吸电子基团的芳族酯和杂芳基酯的还原速度比含有供电子基团的芳基酯更快。NaOMe稳定的NaBH 4溶液的11 B NMR光谱显示较小的四重峰,这是由于单甲氧基硼氢化物(NaBH 3 OMe)在25°C下可持续长达18 h。我们推测NaBH 3 OMe可能是活性还原剂。为支持该假设,计算出氢化物从BH 3(OMe)-转移到苯甲酸甲酯上的活化势垒为18.3 kcal / mol。
更新日期:2018-01-17
中文翻译:

使用催化量的NaOMe稳定甲醇中的NaBH 4。在室温下减少酯和内酯,而不会导致溶剂引起的氢化物损失
NaBH 4与MeOH的快速反应排除了其作为溶剂进行大规模酯还原的用途。我们现已了解到,催化量的NaOMe(5摩尔%)可使NaBH 4溶液在25°C的甲醇中稳定,并允许将这些溶液用于将酯还原为醇类。使用包括天然存在的含手性γ-丁内酯的二羧酸的酯在内的22种酯证明了该还原方法的普遍性。该方法允许在氰基和硝基存在下酯的化学选择性还原,以及吡咯烷二酮酯的还原环化成稠合的五元呋喃[2,3- b]。]吡咯和(-)-crispine A类似物,具有较高的光学和化学收率。内酯,脂族酯,含有吸电子基团的芳族酯和杂芳基酯的还原速度比含有供电子基团的芳基酯更快。NaOMe稳定的NaBH 4溶液的11 B NMR光谱显示较小的四重峰,这是由于单甲氧基硼氢化物(NaBH 3 OMe)在25°C下可持续长达18 h。我们推测NaBH 3 OMe可能是活性还原剂。为支持该假设,计算出氢化物从BH 3(OMe)-转移到苯甲酸甲酯上的活化势垒为18.3 kcal / mol。











































 京公网安备 11010802027423号
京公网安备 11010802027423号