Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis.
The Lancet ( IF 98.4 ) Pub Date : 2018-Feb-01 , DOI: 10.1016/s1470-2045(17)30846-x Julia A Beaver 1 , Maitreyee Hazarika 1 , Flora Mulkey 2 , Sirisha Mushti 2 , Huanyu Chen 2 , Kun He 2 , Rajeshwari Sridhara 2 , Kirsten B Goldberg 1 , Meredith K Chuk 1 , Dow-Chung Chi 1 , Jennie Chang 1 , Amy Barone 1 , Sanjeeve Balasubramaniam 1 , Gideon M Blumenthal 1 , Patricia Keegan 1 , Richard Pazdur 3 , Marc R Theoret 1
The Lancet ( IF 98.4 ) Pub Date : 2018-Feb-01 , DOI: 10.1016/s1470-2045(17)30846-x Julia A Beaver 1 , Maitreyee Hazarika 1 , Flora Mulkey 2 , Sirisha Mushti 2 , Huanyu Chen 2 , Kun He 2 , Rajeshwari Sridhara 2 , Kirsten B Goldberg 1 , Meredith K Chuk 1 , Dow-Chung Chi 1 , Jennie Chang 1 , Amy Barone 1 , Sanjeeve Balasubramaniam 1 , Gideon M Blumenthal 1 , Patricia Keegan 1 , Richard Pazdur 3 , Marc R Theoret 1
Affiliation
|
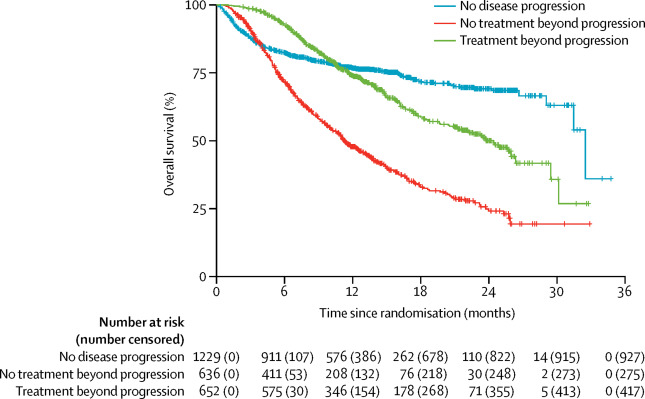
Patients who receive immunotherapeutic drugs might develop an atypical response pattern, wherein they initially meet conventional response criteria for progressive disease but later have decreases in tumour burden. Such responses warrant further investigation into the potential benefits and risks for patients who continue immunotherapy beyond disease progression defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
中文翻译:

使用抗 PD-1 抗体治疗且超出 RECIST 进展的黑色素瘤患者:美国食品和药物管理局的汇总分析。
接受免疫治疗药物的患者可能会出现非典型反应模式,其中他们最初满足疾病进展的常规反应标准,但后来肿瘤负荷减少。这种反应值得进一步研究对于在实体瘤反应评估标准 (RECIST) 1.1 版定义的疾病进展后继续免疫治疗的患者的潜在益处和风险。
更新日期:2018-02-01
中文翻译:

使用抗 PD-1 抗体治疗且超出 RECIST 进展的黑色素瘤患者:美国食品和药物管理局的汇总分析。
接受免疫治疗药物的患者可能会出现非典型反应模式,其中他们最初满足疾病进展的常规反应标准,但后来肿瘤负荷减少。这种反应值得进一步研究对于在实体瘤反应评估标准 (RECIST) 1.1 版定义的疾病进展后继续免疫治疗的患者的潜在益处和风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号