PLOS Biology ( IF 7.8 ) Pub Date : 2018-01-16 , DOI: 10.1371/journal.pbio.2003714 Yu-Ting Chou , Jeng-Kai Jiang , Muh-Hwa Yang , Jeng-Wei Lu , Hua-Kuo Lin , Horng-Dar Wang , Chiou-Hwa Yuh
|
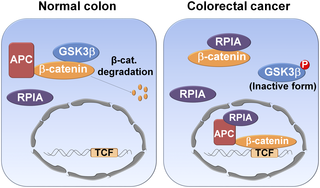
Altered metabolism is one of the hallmarks of cancers. Deregulation of ribose-5-phosphate isomerase A (RPIA) in the pentose phosphate pathway (PPP) is known to promote tumorigenesis in liver, lung, and breast tissues. Yet, the molecular mechanism of RPIA-mediated colorectal cancer (CRC) is unknown. Our study demonstrates a noncanonical function of RPIA in CRC. Data from the mRNAs of 80 patients’ CRC tissues and paired nontumor tissues and protein levels, as well as a CRC tissue array, indicate RPIA is significantly elevated in CRC. RPIA modulates cell proliferation and oncogenicity via activation of β-catenin in colon cancer cell lines. Unlike its role in PPP in which RPIA functions within the cytosol, RPIA enters the nucleus to form a complex with the adenomatous polyposis coli (APC) and β-catenin. This association protects β-catenin by preventing its phosphorylation, ubiquitination, and subsequent degradation. The C-terminus of RPIA (amino acids 290 to 311), a region distinct from its enzymatic domain, is necessary for RPIA-mediated tumorigenesis. Consistent with results in vitro, RPIA increases the expression of β-catenin and its target genes, and induces tumorigenesis in gut-specific promotor-carrying RPIA transgenic zebrafish. Together, we demonstrate a novel function of RPIA in CRC formation in which RPIA enters the nucleus and stabilizes β-catenin activity and suggests that RPIA might be a biomarker for targeted therapy and prognosis.
中文翻译:

核糖5磷酸异构酶A的非规范功能的鉴定通过经由新的C端结构域稳定和激活β-catenin促进结直肠癌的形成
代谢改变是癌症的标志之一。磷酸戊糖途径(PPP)中核糖5-磷酸异构酶A(RPIA)的失调可促进肝,肺和乳腺组织的肿瘤发生。然而,RPIA介导的大肠癌(CRC)的分子机制尚不清楚。我们的研究表明RPIA在CRC中具有非规范功能。来自80位患者CRC组织,成对的非肿瘤组织和蛋白质水平以及CRC组织阵列的mRNA的数据表明,RPIA在CRC中显着升高。RPIA通过激活结肠癌细胞系中的β-catenin调节细胞增殖和致癌性。与RPIA在RPIA在胞质溶胶中发挥作用的PPP中不同,RPIA进入细胞核与腺瘤性息肉病大肠杆菌(APC)和β-catenin形成复合物。该缔合通过防止其磷酸化,泛素化和随后的降解来保护β-catenin。RPIA的C末端(第290至311位氨基酸)是一个不同于其酶促结构域的区域,是RPIA介导的肿瘤发生所必需的。与体外结果一致,RPIA增加了β-catenin及其靶基因的表达,并在携带肠道特异性启动子的RPIA转基因斑马鱼中诱导了肿瘤的发生。在一起,我们证明了RPIA在CRC形成中的新功能,其中RPIA进入细胞核并稳定β-catenin活性,并暗示RPIA可能是靶向治疗和预后的生物标记。与体外结果一致,RPIA增加了β-catenin及其靶基因的表达,并在携带肠道特异性启动子的RPIA转基因斑马鱼中诱导了肿瘤的发生。在一起,我们证明了RPIA在CRC形成中的新功能,其中RPIA进入细胞核并稳定β-catenin活性,并暗示RPIA可能是靶向治疗和预后的生物标记。与体外结果一致,RPIA增加了β-catenin及其靶基因的表达,并在携带肠道特异性启动子的RPIA转基因斑马鱼中诱导了肿瘤的发生。在一起,我们证明了RPIA在CRC形成中的新功能,其中RPIA进入细胞核并稳定β-catenin活性,并暗示RPIA可能是靶向治疗和预后的生物标记。











































 京公网安备 11010802027423号
京公网安备 11010802027423号