当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Aminodiols by Sequential Rhodium‐Catalysed Oxyamination/Kinetic Resolution: Expanding the Substrate Scope of Amidine‐Based Catalysis
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-02-28 , DOI: 10.1002/chem.201705670 Joan Guasch 1 , Irene Giménez‐Nueno 1 , Ignacio Funes‐Ardoiz 2 , Miguel Bernús 1 , M. Isabel Matheu 1 , Feliu Maseras 2, 3 , Sergio Castillón 1 , Yolanda Díaz 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-02-28 , DOI: 10.1002/chem.201705670 Joan Guasch 1 , Irene Giménez‐Nueno 1 , Ignacio Funes‐Ardoiz 2 , Miguel Bernús 1 , M. Isabel Matheu 1 , Feliu Maseras 2, 3 , Sergio Castillón 1 , Yolanda Díaz 1
Affiliation
|
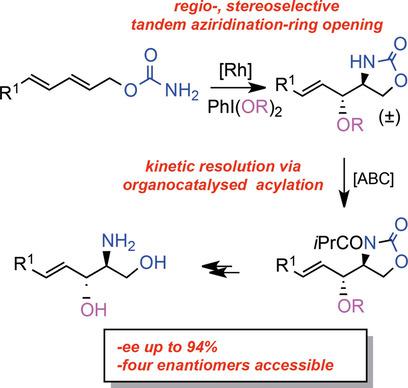
Regio‐ and stereoselective oxyamination of dienes through a tandem rhodium‐catalysed aziridination–nucleophilic opening affords racemic oxazolidinone derivatives, which undergo a kinetic resolution acylation process with amidine‐based catalysts (ABCs) to achieve s values of up to 117. This protocol was applied to the enantioselective synthesis of sphingosine.
中文翻译:

连续铑催化的氧化/动力学拆分对氨基二醇的对映选择性合成:扩大了of基催化的底物范围
通过串联铑催化的叠氮化-亲核开口进行二烯的区域和立体选择性氧化胺化反应,得到外消旋的恶唑烷酮衍生物,其与with基催化剂(ABCs)进行动力学拆分酰化反应,可得到高达117的s值。采用了该协议对鞘氨醇的对映选择性合成。
更新日期:2018-02-28
中文翻译:

连续铑催化的氧化/动力学拆分对氨基二醇的对映选择性合成:扩大了of基催化的底物范围
通过串联铑催化的叠氮化-亲核开口进行二烯的区域和立体选择性氧化胺化反应,得到外消旋的恶唑烷酮衍生物,其与with基催化剂(ABCs)进行动力学拆分酰化反应,可得到高达117的s值。采用了该协议对鞘氨醇的对映选择性合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号