当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Pentagonal‐Pyramidal Hexamethylbenzene Dication: Many Shades of Coordination Chemistry at Carbon
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-03-09 , DOI: 10.1002/chem.201705812 Johannes E. M. N. Klein 1 , Remco W. A. Havenith 2, 3 , Gerald Knizia 4
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-03-09 , DOI: 10.1002/chem.201705812 Johannes E. M. N. Klein 1 , Remco W. A. Havenith 2, 3 , Gerald Knizia 4
Affiliation
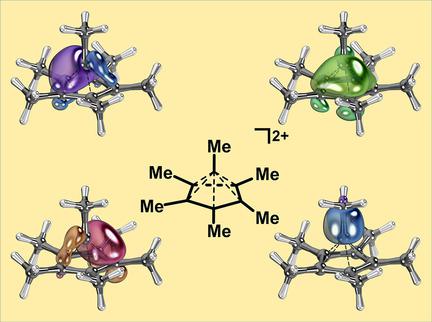
|
A recent report on the crystal structure of the pentagonal‐pyramidal hexamethylbenzene dication C6(CH3)62+ by Malischewski and Seppelt [Angew. Chem. Int. Ed. 2017, 56, 368] confirmed the structural proposal made in the first report of this compound in 1973 by Hogeveen and Kwant [Tetrahedron Lett. 1973, 14, 1665]. The widespread attention that this compound quickly gained led us to reinvestigate its electronic structure. On the basis of intrinsic bond orbital analysis, effective oxidation state analysis, ring current analysis, and comparison with well‐established coordination complexes, it is demonstrated that the central carbon atom behaves like a transition metal. The central (apical) carbon atom, although best described as a highly Lewis‐acidic carbon atom coordinated with an anionic cyclopentadienyl ligand, is also capable of acting as an electron‐pair donor to a formal CH3+ group. The different roles of coordination chemistry are discussed.
中文翻译:

五角形-金字塔形六甲基苯阳离子:碳原子上许多配位化学的阴影
上的晶体结构的最新报告的五边形锥型六甲双阳离子Ç 6(CH 3)6 2+通过Malischewski和Seppelt [ Angew。化学 诠释 埃德。2017年,56,368]证实Hogeveen和Kwant [于1973年在该化合物的第一报告中提出的结构提案四面体通讯。1973年14(1665年)。这种化合物很快引起了广泛的关注,导致我们重新研究了其电子结构。在本征键轨道分析,有效氧化态分析,环电流分析以及与完善的配位化合物进行比较的基础上,证明了中心碳原子的行为类似于过渡金属。中心(顶部)碳原子尽管被描述为与阴离子环戊二烯基配体配位的高路易斯酸性碳原子,但也能够充当正式CH 3 +基团的电子对供体。讨论了配位化学的不同作用。
更新日期:2018-03-09
中文翻译:

五角形-金字塔形六甲基苯阳离子:碳原子上许多配位化学的阴影
上的晶体结构的最新报告的五边形锥型六甲双阳离子Ç 6(CH 3)6 2+通过Malischewski和Seppelt [ Angew。化学 诠释 埃德。2017年,56,368]证实Hogeveen和Kwant [于1973年在该化合物的第一报告中提出的结构提案四面体通讯。1973年14(1665年)。这种化合物很快引起了广泛的关注,导致我们重新研究了其电子结构。在本征键轨道分析,有效氧化态分析,环电流分析以及与完善的配位化合物进行比较的基础上,证明了中心碳原子的行为类似于过渡金属。中心(顶部)碳原子尽管被描述为与阴离子环戊二烯基配体配位的高路易斯酸性碳原子,但也能够充当正式CH 3 +基团的电子对供体。讨论了配位化学的不同作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号