当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of Benzo[b][1,4]oxazins via Intramolecular Trapping Iminoenol
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03804 Hong Zhang 1 , Jinhai Shen 1 , Guolin Cheng 1 , Yadong Feng 1 , Xiuling Cui 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03804 Hong Zhang 1 , Jinhai Shen 1 , Guolin Cheng 1 , Yadong Feng 1 , Xiuling Cui 1
Affiliation
|
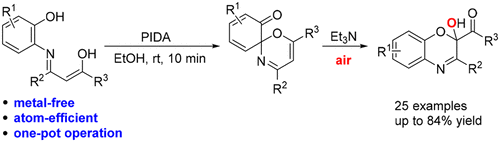
A highly atom-efficient “one-pot” protocol has been developed to construct multisubstituted 2-hydroxy-benzo[b][1,4]oxazins starting from N-(2-hydroxylaryl)enaminones. This procedure comprises a PIDA-mediated intramolecular iminoenol tautomer trapping reaction, followed by Et3N-promoted aerobic oxidative ring construction. In particular, an O2 molecule from air served as the oxygen source of the hydroxyl group in the titled products. This reaction proceeded smoothly at room temperature under air atmosphere and metal-free conditions.
中文翻译:

分子内捕集亚氨基烯醇一锅法合成苯并[ b ] [1,4]恶嗪
已经开发了一种高度原子有效的“一锅”方案,以从N-(2-羟基芳基)烯酮开始构建多取代的2-羟基-苯并[ b ] [1,4]恶嗪。该程序包括PIDA介导的分子内亚氨基烯醇互变异构体捕获反应,然后是Et 3 N-促进的好氧氧化环的构建。特别地,来自空气的O 2分子用作标题产物中羟基的氧源。该反应在室温在空气气氛和无金属的条件下顺利进行。
更新日期:2018-01-17
中文翻译:

分子内捕集亚氨基烯醇一锅法合成苯并[ b ] [1,4]恶嗪
已经开发了一种高度原子有效的“一锅”方案,以从N-(2-羟基芳基)烯酮开始构建多取代的2-羟基-苯并[ b ] [1,4]恶嗪。该程序包括PIDA介导的分子内亚氨基烯醇互变异构体捕获反应,然后是Et 3 N-促进的好氧氧化环的构建。特别地,来自空气的O 2分子用作标题产物中羟基的氧源。该反应在室温在空气气氛和无金属的条件下顺利进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号