当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Based Catalytic Anodes To Produce 2,5-Furandicarboxylic Acid, a Biomass-Derived Alternative to Terephthalic Acid
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acscatal.7b03152 Do-Hwan Nam 1 , Brandon J. Taitt 1 , Kyoung-Shin Choi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acscatal.7b03152 Do-Hwan Nam 1 , Brandon J. Taitt 1 , Kyoung-Shin Choi 1
Affiliation
|
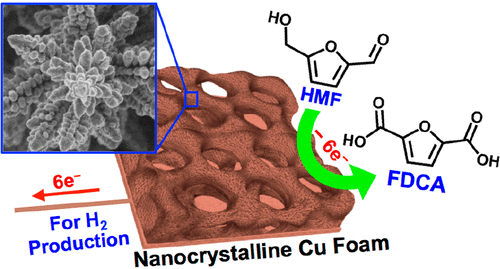
2,5-Furandicarboxylic acid (FDCA) is a key near-market platform chemical that can potentially replace terephthalic acid in various polyesters such as polyethylene terephthalate (PET). FDCA can be obtained from oxidation of 5-hydroxymethylfurfural (HMF), which can be derived from cellulosic biomass through isomerization and dehydration of hexoses. In this study, electrochemical oxidation of HMF to FDCA is demonstrated using Cu, one of the cheapest transition metals, as the catalytic anode. The oxidized Cu surface is not catalytic for water oxidation, which is the major reaction competing with HMF oxidation in aqueous media. Therefore, a wide potential window to oxidize HMF without inducing water oxidation was available, enabling high Faradaic efficiencies for FDCA production. Cu was prepared as nanocrystalline and bulk electrodes by electrodeposition, and key differences in their surface oxidation and electrochemical HMF oxidation were investigated. The oxide and hydroxide layers formed on the nanocrystalline electrode appeared to have an intrinsically different catalytic ability for HMF oxidation from those formed on the bulk electrode. Both the HMF conversion and FDCA production by the nanocrystalline electrode were nearly perfectly proportional to the amount of charge passed with no significant accumulation of any intermediate oxidation product during the course of HMF oxidation. After the stoichiometric amount of charge was passed, the nanocrystalline electrode achieved a FDCA yield of 96.4%. In contrast, the bulk electrode accumulated a significant amount of 5-formyl-2-furancarboxylic acid (FFCA) during HMF oxidation and achieved an FDCA yield of 80.8%. The morphology and composition of the oxide and hydroxide layers formed on the nanocrystalline and bulk electrodes were investigated systematically before and after HMF oxidation.
中文翻译:

铜基催化阳极可生产2,5-呋喃二甲酸,这是对苯二甲酸的生物质替代品
2,5-呋喃二甲酸(FDCA)是一种关键的近端市场平台化学品,可以潜在地替代各种聚酯(例如聚对苯二甲酸乙二醇酯(PET))中的对苯二甲酸。FDCA可以通过5-羟甲基糠醛(HMF)的氧化获得,HMF可以通过己糖的异构化和脱水作用衍生自纤维素生物质。在这项研究中,使用最便宜的过渡金属之一Cu作为催化阳极,证明了HMF电化学氧化为FDCA。氧化的铜表面不能催化水氧化,这是与HMF在水性介质中氧化竞争的主要反应。因此,在不诱导水氧化的情况下,就有广阔的氧化HMF的潜在窗口,从而为FDCA生产提供了很高的法拉第效率。通过电沉积将铜制备为纳米晶和块状电极,并研究了其表面氧化和电化学HMF氧化的关键差异。形成在纳米晶体电极上的氧化物和氢氧化物层似乎具有与形成在体电极上的氧化物固有的HMF氧化催化能力。纳米晶体电极的HMF转化率和FDCA产量均几乎与所通过的电荷成正比,在HMF氧化过程中没有任何中间氧化产物的显着积累。通过化学计量的电荷后,纳米晶电极的FDCA收率为96.4%。相比之下,大块电极在HMF氧化过程中积累了大量的5-甲酰基-2-呋喃甲酸(FFCA),FDCA的产率为80.8%。在HMF氧化之前和之后,系统地研究了在纳米晶体和体电极上形成的氧化物和氢氧化物层的形态和组成。
更新日期:2018-01-17
中文翻译:

铜基催化阳极可生产2,5-呋喃二甲酸,这是对苯二甲酸的生物质替代品
2,5-呋喃二甲酸(FDCA)是一种关键的近端市场平台化学品,可以潜在地替代各种聚酯(例如聚对苯二甲酸乙二醇酯(PET))中的对苯二甲酸。FDCA可以通过5-羟甲基糠醛(HMF)的氧化获得,HMF可以通过己糖的异构化和脱水作用衍生自纤维素生物质。在这项研究中,使用最便宜的过渡金属之一Cu作为催化阳极,证明了HMF电化学氧化为FDCA。氧化的铜表面不能催化水氧化,这是与HMF在水性介质中氧化竞争的主要反应。因此,在不诱导水氧化的情况下,就有广阔的氧化HMF的潜在窗口,从而为FDCA生产提供了很高的法拉第效率。通过电沉积将铜制备为纳米晶和块状电极,并研究了其表面氧化和电化学HMF氧化的关键差异。形成在纳米晶体电极上的氧化物和氢氧化物层似乎具有与形成在体电极上的氧化物固有的HMF氧化催化能力。纳米晶体电极的HMF转化率和FDCA产量均几乎与所通过的电荷成正比,在HMF氧化过程中没有任何中间氧化产物的显着积累。通过化学计量的电荷后,纳米晶电极的FDCA收率为96.4%。相比之下,大块电极在HMF氧化过程中积累了大量的5-甲酰基-2-呋喃甲酸(FFCA),FDCA的产率为80.8%。在HMF氧化之前和之后,系统地研究了在纳米晶体和体电极上形成的氧化物和氢氧化物层的形态和组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号