Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-16 , DOI: 10.1016/j.bioorg.2018.01.020 Bhupinder Kumar , Sheetal , Anil K. Mantha , Vinod Kumar
|
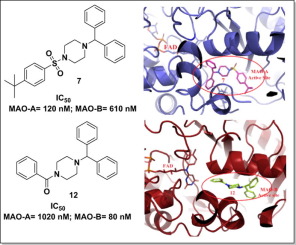
Monoamine oxidase inhibitors (MAOIs) are potential drug candidates for the treatment of various neurological disorders like Parkinson’s disease, Alzheimer’s disease and depression. In the present study, two series of 4-substituted phenylpiperazine and 1-benzhydrylpiperazine (1–21) derivatives were synthesized and screened for their MAO-A and MAO-B inhibitory activity using Amplex Red assay. Most of the synthesized compounds were found selective for MAO-B isoform except compounds 3, 7, 8, 9 and 13 (MAO-A selective) while compound 11 was non-selective. In the current series, compound 12 showed most potent MAO-B inhibitor activity with IC50 value of 80 nM and compound 7 was found to be most potent MAO-A inhibitor with IC50 value of 120 nM and both the compounds were found reversible inhibitors. Compound 8 was found most selective MAO-A inhibitor while compound 20 was found most selective inhibitor for MAO-B isoform. In the cytotoxicity evaluation, all the compounds were found non-toxic to SH-SY5Y and IMR-32 cells at 25 µM concentration. In the ROS studies, compound 8 (MAO-A inhibitor) reduced the ROS level by 51.2% while compound 13 reduced the ROS level by 61.81%. In the molecular dynamic simulation studies for 30 ns, compound 12 was found quite stable in the active cavity of MAO-B. Thus, it can be concluded that phenyl- and 1-benzhydrylpiperazine derivatives are promising MAO inhibitors and can act as a lead to design potent, and selective MAO inhibitors for the treatment of various neurological disorders.
中文翻译:

苯-/苯甲酰基哌嗪衍生物作为潜在的MAO抑制剂的合成,生物学评估和分子模型研究
单胺氧化酶抑制剂(MAOIs)是潜在的候选药物,可用于治疗各种神经系统疾病,如帕金森氏病,阿尔茨海默氏病和抑郁症。在本研究中,两个系列的4-取代苯基哌嗪和1-二苯甲基哌嗪的(1 - 21)衍生物的合成和筛选用的Amplex红测定其MAO-A和MAO-B的抑制活性。大多数合成的化合物被发现为除了化合物MAO-B同种型选择性的3,7,8,9和13(MAO-A选择性),而化合物11是非选择性的。在当前系列中,化合物12表现出最有效的MAO-B抑制剂活性,IC 50值为80 nM,发现化合物7是最有效的MAO-A抑制剂,IC 50值为120 nM,两种化合物均为可逆抑制剂。发现化合物8是最具选择性的MAO-A抑制剂,而化合物20是最具选择性的MAO-B同工型抑制剂。在细胞毒性评估中,发现所有化合物对25 µM浓度的SH-SY5Y和IMR-32细胞无毒。在ROS研究中,化合物8(MAO-A抑制剂)将ROS水平降低了51.2%,而化合物13将ROS水平降低了61.81%。在30 ns的分子动力学模拟研究中,化合物在MAO-B的活动腔中发现有12个非常稳定。因此,可以得出结论,苯基和1-苯甲酰基哌嗪衍生物是有前途的MAO抑制剂,可以作为设计有效的,选择性的MAO抑制剂用于治疗各种神经系统疾病的先导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号