Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2018-01-16 , DOI: 10.1016/j.jcis.2018.01.049 J.M. Rimsza , R.E. Jones , L.J. Criscenti
|
Hypothesis
Sodium adsorption on silica surfaces depends on the solution counter-ion. Here, we use NaOH solutions to investigate basic environments.
Simulations
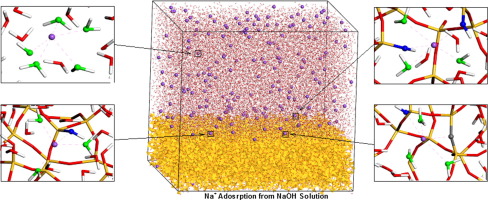
Sodium adsorption on hydroxylated silica surfaces from NaOH solutions were investigated through molecular dynamics with a dissociative force field, allowing for the development of secondary molecular species.
Findings
Across the NaOH concentrations (0.01 M − 1.0 M), ∼50% of the Na+ ions were concentrated in the surface region, developing silica surface charges between − 0.01 C/m2 (0.01 M NaOH) and − 0.76 C/m2 (1.0 M NaOH) due to surface site deprotonation. Five inner-sphere adsorption complexes were identified, including monodentate, bidentate, and tridentate configurations and two additional structures, with Na+ ions coordinated by bridging oxygen and hydroxyl groups or water molecules. Coordination of Na+ ions by bridging oxygen atoms indicates partial or complete incorporation of Na+ ions into the silica surface. Residence time analysis identified that Na+ ions coordinated by bridging oxygen atoms stayed adsorbed onto the surface four times longer than the mono/bi/tridentate species, indicating formation of relatively stable and persistent Na+ ion adsorption structures. Such inner-sphere complexes form only at NaOH concentrations of > 0.5 M. Na+ adsorption and lifetimes have implications for the stability of silica surfaces.
中文翻译:

NaOH溶液与二氧化硅表面的相互作用
假设
钠在二氧化硅表面的吸附取决于溶液的抗衡离子。在这里,我们使用NaOH解决方案来研究基本环境。
模拟
通过具有解离力场的分子动力学研究了钠在NaOH溶液中对羟基化二氧化硅表面的吸附,从而发展了次级分子。
发现
在整个NaOH浓度(0.01 M-1.0 M)中,约50%的Na +离子集中在表面区域,在-0.01 C / m 2(0.01 M NaOH)和-0.76 C / m 2之间形成二氧化硅表面电荷。(1.0 M NaOH)归因于表面位点去质子化。鉴定了五个内层吸附复合物,包括单齿,双齿和三齿构型以及两个其他结构,其中Na +离子通过桥连氧和羟基或水分子来协调。的Na的协调+通过桥接氧原子离子的Na指示的部分或完全掺入+离子进入二氧化硅表面。停留时间分析表明,Na +通过桥接氧原子配位的离子比单/双/三齿物种保持吸附在表面的时间长四倍,表明形成了相对稳定和持久的Na +离子吸附结构。此类内球络合物仅在> 0.5 M的NaOH浓度下形成。Na +的吸附和寿命对二氧化硅表面的稳定性有影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号