当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Enantioselective Total Synthesis of (−)-Petromindole
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.orglett.7b03768 Dattatraya H. Dethe 1 , Susanta Kumar Sau 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.orglett.7b03768 Dattatraya H. Dethe 1 , Susanta Kumar Sau 1
Affiliation
|
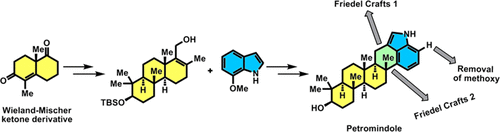
The first enantioselective total synthesis of (−)-petromindole, an architecturally distinct congener of indole diterpene family, has been achieved. Key features of this synthetic route include the scalable and concise synthesis of tricyclic allylic alcohol from enantiopure Wieland–Mischer ketone derivative, and TMSOTf-mediated, highly efficient biomimetic C-4 cyclization of indole derivative for the rapid construction of a hexacyclic skeleton of petromindole.
中文翻译:

(-)-Petromindole的仿生对映选择性全合成
已经实现了对-吲哚并吲哚的第一个对映选择性全合成反应,这是吲哚二萜家族在结构上独特的同类物。该合成路线的关键特征包括从对映纯Wieland-Mischer酮衍生物可扩展且简明的合成三环烯丙基醇,以及TMSOTf介导的吲哚衍生物的高效仿生C-4环化反应,可快速构建邻苯二酚的六环骨架。
更新日期:2018-01-16
中文翻译:

(-)-Petromindole的仿生对映选择性全合成
已经实现了对-吲哚并吲哚的第一个对映选择性全合成反应,这是吲哚二萜家族在结构上独特的同类物。该合成路线的关键特征包括从对映纯Wieland-Mischer酮衍生物可扩展且简明的合成三环烯丙基醇,以及TMSOTf介导的吲哚衍生物的高效仿生C-4环化反应,可快速构建邻苯二酚的六环骨架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号