当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spatiotemporal Control of Pre-existing Alkene Geometry: A Bio-Inspired Route to 4-Trifluoromethyl-2H-chromenes
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.orglett.7b03859 Svenja I. Faßbender 1 , Jan B. Metternich 1 , Ryan Gilmour 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.orglett.7b03859 Svenja I. Faßbender 1 , Jan B. Metternich 1 , Ryan Gilmour 1
Affiliation
|
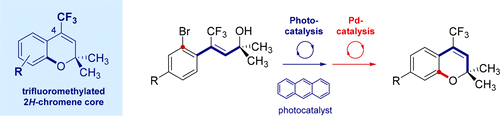
Routes to prepare C4-trifluoromethyl analogues of the 2H-chromene scaffold are scarce: this is particularly striking given the importance of fluorine in pharmaceutical development. To address this limitation, a facile strategy has been developed that is reliant on catalytic, geometric isomerization of easily accessible allylic alcohols (up to >95:5) followed by intramolecular cyclization via Pd catalysis (up to 96%). This concise biomimetic approach emulates the photoisomerization/cyclization cascade inherent to phenylpropanoid biosynthesis.
中文翻译:

时空存在的烯烃几何结构的时空控制:一种生物启发的途径,以4-Trifluoromethyl-2 H -chromenes
制备2 H-亚甲基支架的C4-三氟甲基类似物的途径很少:鉴于氟在药物开发中的重要性,这一点尤其令人震惊。为了解决该限制,已经开发了一种容易的策略,该策略依赖于容易获得的烯丙醇的催化几何异构化(高达> 95:5),然后通过Pd催化进行分子内环化(高达96%)。这种简洁的仿生方法模拟了苯丙烷生物合成固有的光异构化/环化级联反应。
更新日期:2018-01-16
中文翻译:

时空存在的烯烃几何结构的时空控制:一种生物启发的途径,以4-Trifluoromethyl-2 H -chromenes
制备2 H-亚甲基支架的C4-三氟甲基类似物的途径很少:鉴于氟在药物开发中的重要性,这一点尤其令人震惊。为了解决该限制,已经开发了一种容易的策略,该策略依赖于容易获得的烯丙醇的催化几何异构化(高达> 95:5),然后通过Pd催化进行分子内环化(高达96%)。这种简洁的仿生方法模拟了苯丙烷生物合成固有的光异构化/环化级联反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号