当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of Persulfates Using Siderite as a Source of Ferrous Ions: Sulfate Radical Production, Stoichiometric Efficiency, and Implications
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acssuschemeng.7b03948 Yong Feng 1 , Deli Wu 2 , Hailong Li 1, 3 , Jianfeng Bai 4 , Yibo Hu 1 , Changzhong Liao 1, 5 , Xiao-yan Li 1 , Kaimin Shih 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acssuschemeng.7b03948 Yong Feng 1 , Deli Wu 2 , Hailong Li 1, 3 , Jianfeng Bai 4 , Yibo Hu 1 , Changzhong Liao 1, 5 , Xiao-yan Li 1 , Kaimin Shih 1
Affiliation
|
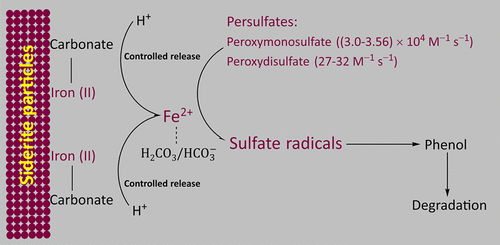
Ferrous ions (Fe2+) rapidly activate persulfates to produce sulfate radicals. However, the high reactivity of Fe2+ toward sulfate radicals means that they are easily scavenged, which reduces the stoichiometric efficiency of persulfates. To improve the stoichiometric efficiency, siderite was used to activate peroxydisulfate (PDS) and peroxymonosulfate (PMS), with phenol as a model contaminant. Near-100% degradation of phenol was achieved by siderite-activated PDS or PMS. In contrast, only 34% and 25% of the phenol was degraded by Fe2+- and nanoscale-magnetite-activated persulfates, respectively. The stoichiometric efficiencies of PMS and PDS activated by siderite were more than 4.4 and 3.6 times higher, respectively, than those activated by Fe2+. Electron paramagnetic resonance recorded both sulfate radicals and hydroxyl radicals. The effects of pH, iron dissolution, and scavenging were characterized, and the results indicated that siderite mainly activated persulfates by acting as a source of Fe2+ and that sulfate radicals were the major active species. The release of Fe2+ and the production of sulfate radicals were controllable via the pH of the solution. No deactivation occurred when the siderite was reused, because the acidic environment partially dissolved the surface. These findings may facilitate the application of iron-bearing materials for sulfate radical production.
中文翻译:

使用菱铁矿作为亚铁离子源的过硫酸盐的活化:硫酸根的产生,化学计量效率及其意义
亚铁离子(Fe 2+)快速活化过硫酸盐以产生硫酸根。然而,Fe 2+对硫酸根的高反应性意味着它们容易被清除,这降低了过硫酸盐的化学计量效率。为了提高化学计量效率,使用菱铁矿活化过氧二硫酸盐(PDS)和过氧一硫酸盐(PMS),以苯酚为模型污染物。通过菱铁矿活化的PDS或PMS,苯酚的降解率接近100%。相比之下,分别只有34%和25%的苯酚被Fe 2+和纳米级磁铁矿活化的过硫酸盐降解。菱铁矿活化的PMS和PDS的化学计量效率分别比Fe 2+活化的化学计量效率高4.4倍和3.6倍。电子顺磁共振记录了硫酸根和羟基。表征了pH,铁溶解和清除的影响,结果表明菱铁矿主要通过作为Fe 2+的来源活化过硫酸盐,而硫酸根是主要的活性物质。通过溶液的pH可控制Fe 2+的释放和硫酸根的产生。重用菱铁矿时不会发生失活,因为酸性环境会部分溶解表面。这些发现可能促进含铁材料在硫酸根自由基生产中的应用。
更新日期:2018-01-16
中文翻译:

使用菱铁矿作为亚铁离子源的过硫酸盐的活化:硫酸根的产生,化学计量效率及其意义
亚铁离子(Fe 2+)快速活化过硫酸盐以产生硫酸根。然而,Fe 2+对硫酸根的高反应性意味着它们容易被清除,这降低了过硫酸盐的化学计量效率。为了提高化学计量效率,使用菱铁矿活化过氧二硫酸盐(PDS)和过氧一硫酸盐(PMS),以苯酚为模型污染物。通过菱铁矿活化的PDS或PMS,苯酚的降解率接近100%。相比之下,分别只有34%和25%的苯酚被Fe 2+和纳米级磁铁矿活化的过硫酸盐降解。菱铁矿活化的PMS和PDS的化学计量效率分别比Fe 2+活化的化学计量效率高4.4倍和3.6倍。电子顺磁共振记录了硫酸根和羟基。表征了pH,铁溶解和清除的影响,结果表明菱铁矿主要通过作为Fe 2+的来源活化过硫酸盐,而硫酸根是主要的活性物质。通过溶液的pH可控制Fe 2+的释放和硫酸根的产生。重用菱铁矿时不会发生失活,因为酸性环境会部分溶解表面。这些发现可能促进含铁材料在硫酸根自由基生产中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号