Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2018-01-16 , DOI: 10.1016/j.micromeso.2018.01.010 S. Hadi Madani , Phillip Kwong , Francisco Rodríguez-Reinoso , Mark J. Biggs , Phillip Pendleton
|
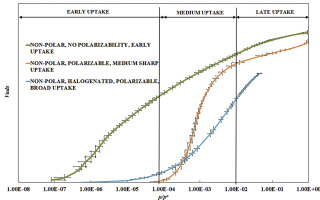
A suite of non-polar adsorptives of different kinetic diameters and shape were used to determine adsorption and pore filling mechanism of a well-characterised poly(furfuryl alcohol)-based activated carbon. Triplicate measured Type I adsorption isotherms for each adsorptive were averaged to provide standard deviation in relative pressures and associated amounts adsorbed. Plateau amounts adsorbed for N2, Ar, CH4, and C6H6, provided Gurvitsch volumes averaged to 0.368 ± 0.015 cm3(liq)/g. The calculated Gurvitsch volumes were compared with those derived via the Dubinin-Radushkevich (DR) equation. Additional adsorptives were CO2, iso-butane and SF6. The results of these 7 adsorptives were used to qualitatively analyse and decode a micropore filling adsorption mechanism. The DR equation was also used for further analysis of the pore filling mechanism. Based on the adsorbate-adsorbate and adsorbate-adsorbent interactions, adsorbates were classified into three groups: (a) Non-polar with non-specific interactions (no dipole, no quadrupole, not readily polarizable: Ar, N2, CH4 and iso-butane), adsorbing as a continuous uptake over the observed relative pressure range; (b) Non-polar adsorptives with potential for specific interactions (no dipole, quadrupole moment: CO2 and C6H6), adsorbing as a condensation process over a relatively narrow relative pressure range in a medium pressure range; (c) Halogenated adsorptives (no dipole, no quadrupole, polarizable: SF6), adsorbing with an S-shaped uptake extending over a relatively broad relative pressure range.
中文翻译:

解码气固相互作用对吸附等温线形状的影响:I.非极性吸附剂
使用一组具有不同动力学直径和形状的非极性吸附剂来确定具有良好特征的基于聚糠醇的活性炭的吸附和孔填充机理。对每种吸附剂一式三份测得的I型吸附等温线取平均值,以提供相对压力和相关吸附量的标准偏差。如果Gurvitsch体积平均为0.368±0.015 cm 3(liq)/ g ,则对于N 2,Ar,CH 4和C 6 H 6吸附的平稳量。将计算出的Gurvitsch体积与通过Dubinin-Radushkevich(DR)公式得出的体积进行比较。其他吸附剂为CO 2,异丁烷和SF 6。这7种吸附剂的结果用于定性分析和解释微孔填充吸附机理。DR方程还用于进一步分析孔隙填充机理。根据被吸附物-被吸附物和被吸附物-吸附剂之间的相互作用,将被吸附物分为三类:(a)具有非特异性相互作用的非极性物质(无偶极,无四极子,不易极化:Ar,N 2,CH 4和iso -丁烷),在观察到的相对压力范围内连续吸收;(b)具有特定相互作用潜能的非极性吸附剂(无偶极,四极矩:CO 2和C 6 H 6),在中等压力范围内相对狭窄的相对压力范围内作为冷凝过程进行吸附;(c)卤化吸附剂(无偶极,无四极,可极化:SF 6),吸收呈S形,吸收范围相对较大。









































 京公网安备 11010802027423号
京公网安备 11010802027423号