当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
96 weeks treatment of tenofovir alafenamide vs . tenofovir disoproxil fumarate for hepatitis B virus infection
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.jhep.2017.11.039 Kosh Agarwal , Maurizia Brunetto , Wai Kay Seto , Young-Suk Lim , Scott Fung , Patrick Marcellin , Sang Hoon Ahn , Namiki Izumi , Wan–Long Chuang , Ho Bae , Manoj Sharma , Harry L.A. Janssen , Calvin Q. Pan , Mustafa Kemal Çelen , Norihiro Furusyo , Dr. Shalimar , Ki Tae Yoon , Huy Trinh , John F. Flaherty , Anuj Gaggar , Audrey H. Lau , Andrea L. Cathcart , Lanjia Lin , Neeru Bhardwaj , Vithika Suri , G. Mani Subramanian , Edward J. Gane , Maria Buti , Henry L.Y. Chan
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.jhep.2017.11.039 Kosh Agarwal , Maurizia Brunetto , Wai Kay Seto , Young-Suk Lim , Scott Fung , Patrick Marcellin , Sang Hoon Ahn , Namiki Izumi , Wan–Long Chuang , Ho Bae , Manoj Sharma , Harry L.A. Janssen , Calvin Q. Pan , Mustafa Kemal Çelen , Norihiro Furusyo , Dr. Shalimar , Ki Tae Yoon , Huy Trinh , John F. Flaherty , Anuj Gaggar , Audrey H. Lau , Andrea L. Cathcart , Lanjia Lin , Neeru Bhardwaj , Vithika Suri , G. Mani Subramanian , Edward J. Gane , Maria Buti , Henry L.Y. Chan
|
BACKGROUND & AIMS
Tenofovir alafenamide (TAF) is a new prodrug of tenofovir developed to treat patients with chronic hepatitis B virus (HBV) infection at a lower dose than tenofovir disoproxil fumarate (TDF) through more efficient delivery of tenofovir to hepatocytes. In 48-week results from two ongoing, double-blind, randomized phase III trials, TAF was non-inferior to TDF in efficacy with improved renal and bone safety. We report 96-week outcomes for both trials. METHODS
In two international trials, patients with chronic HBV infection were randomized 2:1 to receive 25 mg TAF or 300 mg TDF in a double-blinded fashion. One study enrolled HBeAg-positive patients and the other HBeAg-negative patients. We assessed efficacy in each study, and safety in the pooled population. RESULTS
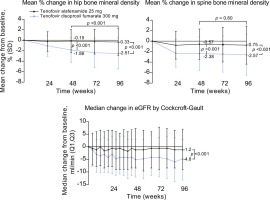
At week 96, the differences in the rates of viral suppression were similar in HBeAg-positive patients receiving TAF and TDF (73% vs. 75%, respectively, adjusted difference -2.2% (95% CI -8.3 to 3.9%; p = 0.47), and in HBeAg-negative patients receiving TAF and TDF (90% vs. 91%, respectively, adjusted difference -0.6% (95% CI -7.0 to 5.8%; p = 0.84). In both studies the proportions of patients with alanine aminotransferase above the upper limit of normal at baseline, who had normal alanine aminotransferase at week 96 of treatment, were significantly higher in patients receiving TAF than in those receiving TDF. In the pooled safety population, patients receiving TAF had significantly smaller decreases in bone mineral density than those receiving TDF in the hip (mean % change -0.33% vs. -2.51%; p <0.001) and lumbar spine (mean % change -0.75% vs. -2.57%; p <0.001), as well as a significantly smaller median change in estimated glomerular filtration rate by Cockcroft-Gault method (-1.2 vs. -4.8 mg/dl; p <0.001). CONCLUSION
In patients with HBV infection, TAF remained as effective as TDF, with continued improved renal and bone safety, two years after the initiation of treatment. Clinicaltrials.gov number: NCT01940471 and NCT01940341. LAY SUMMARY
At week 96 of two ongoing studies comparing the efficacy and safety of tenofovir alafenamide (TAF) to tenofovir disoproxil fumarate (TDF) for the treatment of chronic hepatitis B patients, TAF continues to be as effective as TDF with continued improved renal and bone safety. Registration: Clinicaltrials.gov number: NCT01940471 and NCT01940341.
中文翻译:

替诺福韦艾拉酚胺治疗 96 周 vs . 富马酸替诺福韦二吡呋酯治疗乙型肝炎病毒感染
背景与目的 替诺福韦艾拉酚胺 (TAF) 是一种新的替诺福韦前药,旨在通过更有效地将替诺福韦递送至肝细胞,以比富马酸替诺福韦酯 (TDF) 更低的剂量治疗慢性乙型肝炎病毒 (HBV) 感染患者。在两项正在进行的、双盲、随机的 III 期试验的 48 周结果中,TAF 在疗效方面不劣于 TDF,并提高了肾脏和骨骼的安全性。我们报告了两项试验的 96 周结果。方法 在两项国际试验中,慢性 HBV 感染患者以双盲方式以 2:1 的比例随机接受 25 mg TAF 或 300 mg TDF。一项研究招募了 HBeAg 阳性患者和其他 HBeAg 阴性患者。我们评估了每项研究的有效性以及合并人群的安全性。结果 在第 96 周,p <0.001),以及通过 Cockcroft-Gault 方法估计的肾小球滤过率的中值变化显着更小(-1.2 与 -4.8 mg/dl;p <0.001)。结论 在 HBV 感染患者中,TAF 仍然与 TDF 一样有效,并且在开始治疗两年后肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦二吡呋酯 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。以及通过 Cockcroft-Gault 方法估计的肾小球滤过率的中值变化显着更小(-1.2 与 -4.8 mg/dl;p <0.001)。结论 在 HBV 感染患者中,TAF 仍然与 TDF 一样有效,并且在开始治疗两年后肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦二吡呋酯 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。以及通过 Cockcroft-Gault 方法估计的肾小球滤过率的中值变化显着更小(-1.2 与 -4.8 mg/dl;p <0.001)。结论 在 HBV 感染患者中,TAF 仍然与 TDF 一样有效,并且在开始治疗两年后肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦二吡呋酯 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。在开始治疗两年后,肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦地索普西 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。在开始治疗两年后,肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦地索普西 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。TAF 继续与 TDF 一样有效,并持续提高肾脏和骨骼安全性。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。TAF 继续与 TDF 一样有效,并持续提高肾脏和骨骼安全性。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。
更新日期:2018-04-01
中文翻译:

替诺福韦艾拉酚胺治疗 96 周 vs . 富马酸替诺福韦二吡呋酯治疗乙型肝炎病毒感染
背景与目的 替诺福韦艾拉酚胺 (TAF) 是一种新的替诺福韦前药,旨在通过更有效地将替诺福韦递送至肝细胞,以比富马酸替诺福韦酯 (TDF) 更低的剂量治疗慢性乙型肝炎病毒 (HBV) 感染患者。在两项正在进行的、双盲、随机的 III 期试验的 48 周结果中,TAF 在疗效方面不劣于 TDF,并提高了肾脏和骨骼的安全性。我们报告了两项试验的 96 周结果。方法 在两项国际试验中,慢性 HBV 感染患者以双盲方式以 2:1 的比例随机接受 25 mg TAF 或 300 mg TDF。一项研究招募了 HBeAg 阳性患者和其他 HBeAg 阴性患者。我们评估了每项研究的有效性以及合并人群的安全性。结果 在第 96 周,p <0.001),以及通过 Cockcroft-Gault 方法估计的肾小球滤过率的中值变化显着更小(-1.2 与 -4.8 mg/dl;p <0.001)。结论 在 HBV 感染患者中,TAF 仍然与 TDF 一样有效,并且在开始治疗两年后肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦二吡呋酯 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。以及通过 Cockcroft-Gault 方法估计的肾小球滤过率的中值变化显着更小(-1.2 与 -4.8 mg/dl;p <0.001)。结论 在 HBV 感染患者中,TAF 仍然与 TDF 一样有效,并且在开始治疗两年后肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦二吡呋酯 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。以及通过 Cockcroft-Gault 方法估计的肾小球滤过率的中值变化显着更小(-1.2 与 -4.8 mg/dl;p <0.001)。结论 在 HBV 感染患者中,TAF 仍然与 TDF 一样有效,并且在开始治疗两年后肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦二吡呋酯 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。在开始治疗两年后,肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦地索普西 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。在开始治疗两年后,肾脏和骨骼安全性持续改善。Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。LAY 总结 在比较替诺福韦艾拉酚胺 (TAF) 与富马酸替诺福韦地索普西 (TDF) 治疗慢性乙型肝炎患者的疗效和安全性的两项正在进行的研究的第 96 周时,TAF 继续与 TDF 一样有效,并持续改善肾脏和骨骼安全。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。TAF 继续与 TDF 一样有效,并持续提高肾脏和骨骼安全性。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。TAF 继续与 TDF 一样有效,并持续提高肾脏和骨骼安全性。注册:Clinicaltrials.gov 编号:NCT01940471 和 NCT01940341。











































 京公网安备 11010802027423号
京公网安备 11010802027423号