当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Factors Affecting Binding of Platinum Anticancer Agents with Phospholipids: Influence of Charge and Phosphate Clamp Formation
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-02-28 , DOI: 10.1002/chem.201705822 Anil Kumar Gorle 1 , Junyong Zhang 2, 3 , Qin Liu 4, 5 , Susan J. Berners‐Price 1, 2 , Nicholas P. Farrell 1, 4
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-02-28 , DOI: 10.1002/chem.201705822 Anil Kumar Gorle 1 , Junyong Zhang 2, 3 , Qin Liu 4, 5 , Susan J. Berners‐Price 1, 2 , Nicholas P. Farrell 1, 4
Affiliation
|
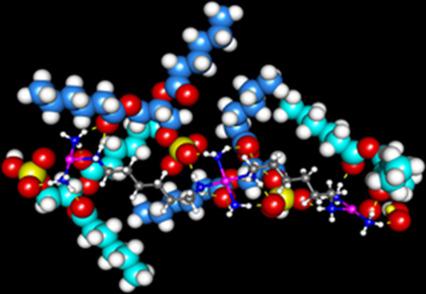
We report a detailed NMR and DFT study of the interaction of polynuclear platinum anticancer agents (PPCs) with negatively charged phospholipids as a mechanism for their cellular uptake. The reactions of fully 15N‐labelled [{trans‐PtCl(NH3)2}2(μ‐trans‐Pt(NH3)2{NH2(CH2)6NH2}2)]4+ (15N‐1, 1,0,1/t,t,t) and the dinuclear [{trans‐PtCl(NH3)2}2{μ‐H2N(CH2)6NH2}]2+ (15N‐2, 1,1/t,t) with the sodium salt of 1,2‐dihexanoyl‐sn‐glycero‐3‐phosphate (DHPA) were studied at 298 K, pH ≈5.4, by [1H,15N] HSQC 2D NMR spectroscopy. Both 15N‐1 and 15N‐2 form an initial mono‐adduct in which the DHPA is coordinated via the phosphate O atom. For the dinuclear 15N‐2, coordination of a second DHPA, in two different orientations, leads to two conformers of the bifunctional adduct. For 15N‐1, coordination of the second DHPA allows the central {PtN4} coordination unit to bind electrostatically to two additional DHPA molecules via phosphate clamp interactions, in an extended network. For both 1,0,1/t,t,t (1) and 1,1/t,t (2), equilibrium conditions are obtained more slowly (>35 h) than in the presence of phosphate (12 h) and in each case the rate constant for the first step of DHPA binding (kL) is about 8 times higher than that for phosphate, whereas the rate constants for the reverse reactions are quite similar. Reaction of 15N‐1 with the sodium salt of 1,2‐dihexanoyl‐sn‐glycero‐3‐[phosphatidyl‐l‐serine] (DHPS) showed only minor adduct formation via coordination to the N‐donor atom of the phosphoserine group.
中文翻译:

影响铂抗癌剂与磷脂结合的结构因素:电荷和磷酸盐钳形成的影响。
我们报告了多核铂抗癌剂(PPC)与带负电荷的磷脂相互作用作为其细胞摄取机制的详细的NMR和DFT研究。完全15 N标记的[{反-PtCl(NH 3)2 } 2(μ反-Pt(NH 3)2 {NH 2(CH 2)6 NH 2 } 2)] 4+(15 N - 1,1,0,1 / T,T,T)和双核[{反式-PtCl(NH 3)2} 2 {μ-H 2 N(CH 2)6 NH 2 }] 2+(15 N- 2,1,1 / T,T)与的钠盐1,2- dihexanoyl- SN -glycero -3-通过[ 1 H,15 N] HSQC 2D NMR光谱研究了磷酸盐(DHPA)在298 K,pH≈5.4时的情况。两个15 N- 1和15 N- 2形式的初始单加合物,其中所述DHPA经由磷酸氧原子配位。对于双核15 N- 2,第二DHPA在两个不同方向上的配位,导致双功能加合物的两个构象异构体。对于15 N- 1,第二个DHPA的配位使中央{PtN 4 }配位单元在扩展的网络中通过磷酸盐钳位相互作用与两个其他DHPA分子静电结合。对于1,0,1 / t,t,t(1)和1,1 / t,t(2)而言,获得平衡条件的时间要比在磷酸盐存在下(12 h)和磷酸盐存在下(32 h)慢得多(> 35 h)。在每种情况下,DHPA结合第一步的速率常数(k L)约为磷酸盐的8倍,而逆反应的速率常数则非常相似。的反应15 N- 1与的钠盐1,2- dihexanoyl- SN -glycero -3- [磷脂酰-升-丝氨酸](DHPS)显示经由协调只有轻微的加合物形成的Ñ的磷酸丝氨酸组的端供体原子。
更新日期:2018-02-28
中文翻译:

影响铂抗癌剂与磷脂结合的结构因素:电荷和磷酸盐钳形成的影响。
我们报告了多核铂抗癌剂(PPC)与带负电荷的磷脂相互作用作为其细胞摄取机制的详细的NMR和DFT研究。完全15 N标记的[{反-PtCl(NH 3)2 } 2(μ反-Pt(NH 3)2 {NH 2(CH 2)6 NH 2 } 2)] 4+(15 N - 1,1,0,1 / T,T,T)和双核[{反式-PtCl(NH 3)2} 2 {μ-H 2 N(CH 2)6 NH 2 }] 2+(15 N- 2,1,1 / T,T)与的钠盐1,2- dihexanoyl- SN -glycero -3-通过[ 1 H,15 N] HSQC 2D NMR光谱研究了磷酸盐(DHPA)在298 K,pH≈5.4时的情况。两个15 N- 1和15 N- 2形式的初始单加合物,其中所述DHPA经由磷酸氧原子配位。对于双核15 N- 2,第二DHPA在两个不同方向上的配位,导致双功能加合物的两个构象异构体。对于15 N- 1,第二个DHPA的配位使中央{PtN 4 }配位单元在扩展的网络中通过磷酸盐钳位相互作用与两个其他DHPA分子静电结合。对于1,0,1 / t,t,t(1)和1,1 / t,t(2)而言,获得平衡条件的时间要比在磷酸盐存在下(12 h)和磷酸盐存在下(32 h)慢得多(> 35 h)。在每种情况下,DHPA结合第一步的速率常数(k L)约为磷酸盐的8倍,而逆反应的速率常数则非常相似。的反应15 N- 1与的钠盐1,2- dihexanoyl- SN -glycero -3- [磷脂酰-升-丝氨酸](DHPS)显示经由协调只有轻微的加合物形成的Ñ的磷酸丝氨酸组的端供体原子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号