当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Selectivity in Aliphatic C−H Oxidation through Supramolecular Recognition
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-03-08 , DOI: 10.1002/chem.201704852 Diego Vidal 1 , Giorgio Olivo 1 , Miquel Costas 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-03-08 , DOI: 10.1002/chem.201704852 Diego Vidal 1 , Giorgio Olivo 1 , Miquel Costas 1
Affiliation
|
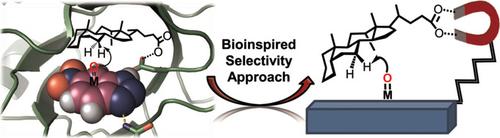
Aliphatic C−H oxidation is the most straightforward approach to functionalize hydrocarbon skeletons. The main challenge of this reaction is the control of site selectivity, given the multiple C−H bonds present in any organic molecule. Natural enzymes elegantly solve this problem through the interplay of different interactions that geometrically orient the substrate to expose a single C−H bond to the active unit, thus overriding intrinsic reactivity patterns. A combination of molecular catalysts and supramolecular receptors can be a promising way to replicate such control. This strategy indeed unlocks hydroxylation of C−H bonds that are not accessible with conventional methodologies, in which the selectivity is dictated by the geometry of the substrate–receptor adduct. Herein, we review the reports of recognition‐driven C−H oxidation reactions and highlight the key design principles that inspired these works.
中文翻译:

通过超分子识别控制脂肪CH氧化中的选择性
脂肪族CH氧化是使烃骨架官能化的最直接方法。考虑到任何有机分子中存在多个CH键,该反应的主要挑战是对位点选择性的控制。天然酶通过不同相互作用的相互作用优雅地解决了这个问题,这些相互作用使底物在几何上定向以使单个CH键暴露于活性单元,从而覆盖了固有的反应性模式。分子催化剂和超分子受体的组合可能是重复进行这种控制的有前途的方法。这种策略的确能解锁常规方法无法获得的CH键的羟基化作用,其中选择性由底物-受体加合物的几何形状决定。在此处,
更新日期:2018-03-08
中文翻译:

通过超分子识别控制脂肪CH氧化中的选择性
脂肪族CH氧化是使烃骨架官能化的最直接方法。考虑到任何有机分子中存在多个CH键,该反应的主要挑战是对位点选择性的控制。天然酶通过不同相互作用的相互作用优雅地解决了这个问题,这些相互作用使底物在几何上定向以使单个CH键暴露于活性单元,从而覆盖了固有的反应性模式。分子催化剂和超分子受体的组合可能是重复进行这种控制的有前途的方法。这种策略的确能解锁常规方法无法获得的CH键的羟基化作用,其中选择性由底物-受体加合物的几何形状决定。在此处,











































 京公网安备 11010802027423号
京公网安备 11010802027423号