当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Etoricoxib in Aqueous Solutions of Glycerin, Methanol, Polyethylene Glycols 200, 400, 600, and Propylene Glycol at 298.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jced.7b00709 Pavan B. Rathi 1, 2 , Mayura Kale 3 , Jafar Soleymani 4 , Abolghasem Jouyban 5, 6
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jced.7b00709 Pavan B. Rathi 1, 2 , Mayura Kale 3 , Jafar Soleymani 4 , Abolghasem Jouyban 5, 6
Affiliation
|
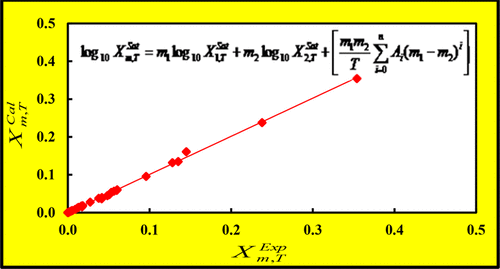
Experimental molar solubility of etoricoxib (ETR) in monosolvents such as glycerin, methanol (MeOH), polyethylene glycol 200 (PEG 200), polyethylene glycol 400 (PEG 400), polyethylene glycol 600 (PEG 600), propylene glycol (PG), and their aqueous binary solvent systems in various mass fraction compositions along with the solute-free and saturated solution densities were measured at 298.2 K. The resulting mole fraction solubility and density data were further correlated and predicted with the Jouyban–Acree model. Overall mean percentage deviations (OMPDs) between experimental and calculated mole fraction solubilities were 3.5%. The solute-free density of the monosolvents and their aqueous binary solvent systems were employed to train the model and then the densities of the saturated solutions were predicted. Moreover, OMPDs for back calculated solute-free densities and predicted saturated solution densities were 0.07% and 0.40%, respectively. Thus, the Jouyban–Acree model have potential use in preformulation and formulation studies during which solubility and density calculations are important physicochemical properties for design and development of new drug products in pharmaceutical industries. The simulated data could also be employed in crystallization and other related process design in the pharmaceutical/chemical industry.
中文翻译:

依托考昔在甘油,甲醇,聚乙二醇200、400、600和丙二醇在298.2 K的水溶液中的溶解度
依托昔布(ETR)在甘油,甲醇(MeOH),聚乙二醇200(PEG 200),聚乙二醇400(PEG 400),聚乙二醇600(PEG 600),丙二醇(PG)和在298.2 K下测量了它们在各种质量分数组成中的二元水溶液体系以及无溶质和饱和溶液的密度。得到的摩尔分数溶解度和密度数据进一步关联起来,并使用Jouyban–Acree模型进行了预测。实验和计算的摩尔分数溶解度之间的总体平均百分比偏差(OMPDs)为3.5%。使用单溶剂及其水溶液二元溶剂体系的无溶质密度训练模型,然后预测饱和溶液的密度。而且,反向计算的无溶质密度和预测的饱和溶液密度的OMPD分别为0.07%和0.40%。因此,Jouyban-Acree模型在预制剂和制剂研究中具有潜在用途,在此期间,溶解度和密度计算对于制药行业新药产品的设计和开发是重要的理化性质。模拟数据也可用于制药/化学行业的结晶和其他相关工艺设计中。Jouyban–Acree模型在预制剂和制剂研究中具有潜在用途,在此期间,溶解度和密度计算对于制药行业新药产品的设计和开发是重要的理化性质。模拟数据也可用于制药/化学行业的结晶和其他相关工艺设计中。Jouyban–Acree模型在预制剂和制剂研究中具有潜在用途,在此期间,溶解度和密度计算对于制药行业新药产品的设计和开发是重要的理化性质。模拟数据也可用于制药/化学行业的结晶和其他相关工艺设计中。
更新日期:2018-01-16
中文翻译:

依托考昔在甘油,甲醇,聚乙二醇200、400、600和丙二醇在298.2 K的水溶液中的溶解度
依托昔布(ETR)在甘油,甲醇(MeOH),聚乙二醇200(PEG 200),聚乙二醇400(PEG 400),聚乙二醇600(PEG 600),丙二醇(PG)和在298.2 K下测量了它们在各种质量分数组成中的二元水溶液体系以及无溶质和饱和溶液的密度。得到的摩尔分数溶解度和密度数据进一步关联起来,并使用Jouyban–Acree模型进行了预测。实验和计算的摩尔分数溶解度之间的总体平均百分比偏差(OMPDs)为3.5%。使用单溶剂及其水溶液二元溶剂体系的无溶质密度训练模型,然后预测饱和溶液的密度。而且,反向计算的无溶质密度和预测的饱和溶液密度的OMPD分别为0.07%和0.40%。因此,Jouyban-Acree模型在预制剂和制剂研究中具有潜在用途,在此期间,溶解度和密度计算对于制药行业新药产品的设计和开发是重要的理化性质。模拟数据也可用于制药/化学行业的结晶和其他相关工艺设计中。Jouyban–Acree模型在预制剂和制剂研究中具有潜在用途,在此期间,溶解度和密度计算对于制药行业新药产品的设计和开发是重要的理化性质。模拟数据也可用于制药/化学行业的结晶和其他相关工艺设计中。Jouyban–Acree模型在预制剂和制剂研究中具有潜在用途,在此期间,溶解度和密度计算对于制药行业新药产品的设计和开发是重要的理化性质。模拟数据也可用于制药/化学行业的结晶和其他相关工艺设计中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号