当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Behavior of Imidazolium-Based Ionic Liquid + Phosphonium-Based Ionic Liquid Mixtures with a Common Anion: Effects of the Alkyl-Chain Length of Cation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jced.7b00808 Takuya Shimomura 1 , Masakazu Sugiyama 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jced.7b00808 Takuya Shimomura 1 , Masakazu Sugiyama 1
Affiliation
|
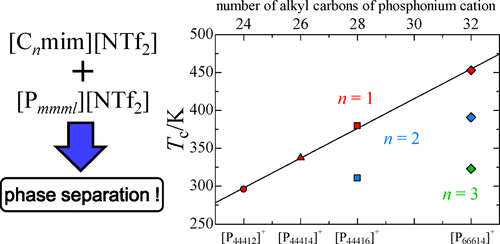
The phase behavior of imidazolium-based ionic liquid (IL) + phosphonium-based IL binary mixtures with a common anion has been investigated in the temperature range 253–453 K. Bis(trifluoromethanesulfonyl)amide ([NTf2]−) and tetrafluoroborate ([BF4]−) were selected as common anions. The imidazolium-based IL + phosphonium-based IL mixtures with a common anion showed an upper critical solution temperature (UCST). The phase separation temperatures of the mixtures decreased with increasing alkyl-chain length of the imidazolium cation. In contrast, the phase separation temperatures of the mixtures increased as the alkyl-chain length of the phosphonium cation increased. The phase separation temperatures of the imidazolium-based IL + phosphonium-based IL mixtures with [BF4]− as a common anion were remarkably higher than those with [NTf2]−.
中文翻译:

咪唑基离子液体+磷基离子液体与常见阴离子的相行为:阳离子的烷基链长度的影响
在253–453 K的温度范围内,研究了咪唑基离子液体(IL)+ nium基IL二元混合物与常见阴离子的相行为。双(三氟甲磺酰基)酰胺([NTf 2 ] -)和四氟硼酸酯( [BF 4 ] -)被选为常见阴离子。基于咪唑鎓的IL +基于磷鎓的IL与常见阴离子的混合物显示出较高的临界溶液温度(UCST)。混合物的相分离温度随着咪唑阳离子的烷基链长度的增加而降低。相反,混合物的相分离温度随着the阳离子的烷基链长度的增加而增加。具有[BF 4 ] -作为常见阴离子的咪唑基IL + IL基IL混合物的相分离温度明显高于具有[NTf 2 ] -的咪唑基IL + IL基IL混合物的相分离温度。
更新日期:2018-01-16
中文翻译:

咪唑基离子液体+磷基离子液体与常见阴离子的相行为:阳离子的烷基链长度的影响
在253–453 K的温度范围内,研究了咪唑基离子液体(IL)+ nium基IL二元混合物与常见阴离子的相行为。双(三氟甲磺酰基)酰胺([NTf 2 ] -)和四氟硼酸酯( [BF 4 ] -)被选为常见阴离子。基于咪唑鎓的IL +基于磷鎓的IL与常见阴离子的混合物显示出较高的临界溶液温度(UCST)。混合物的相分离温度随着咪唑阳离子的烷基链长度的增加而降低。相反,混合物的相分离温度随着the阳离子的烷基链长度的增加而增加。具有[BF 4 ] -作为常见阴离子的咪唑基IL + IL基IL混合物的相分离温度明显高于具有[NTf 2 ] -的咪唑基IL + IL基IL混合物的相分离温度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号