当前位置:
X-MOL 学术
›
Toxicol. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Case examples of an evaluation of the human relevance of the pyrethroids/pyrethrins-induced liver tumours in rodents based on the mode of action†
Toxicology Research ( IF 2.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1039/c7tx00288b Tomoya Yamada 1, 2, 3, 4, 5
Toxicology Research ( IF 2.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1039/c7tx00288b Tomoya Yamada 1, 2, 3, 4, 5
Affiliation
|
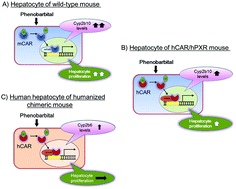
Rodent carcinogenicity studies are useful for screening for human carcinogens but they are not perfect. Some modes of action (MOAs) lead to cancers in both experimental rodents and humans, but others that lead to cancers in rodents do not do so in humans. Therefore, analysing the MOAs by which chemicals produce tumours in rodents and determining the relevance of such tumour data for human risk are critical. Recently, experimental data were obtained as case examples of an evaluation of the human relevance of pyrethroid (metofluthrin and momfluorothrin)- and pyrethrins-induced liver tumours in rats based on MOA. The MOA analysis, based on the International Programme on Chemical Safety (IPCS) framework, concluded that experimental data strongly support that the postulated MOA for metofluthrin-, momfluorothrin- and pyrethrins-produced rat hepatocellular tumours is mediated by constitutive androstane receptor (CAR) activation. Since metofluthrin and momfluorothrin are close structural analogues, reproducible outcomes for both chemicals provide confidence in the MOA findings. Furthermore, cultured human hepatocyte studies and humanized chimeric mouse liver studies demonstrated species difference between human hepatocytes (refractory to the mitogenic effects of these compounds) and rat hepatocytes (sensitive to their mitogenic effects). These data strongly support the hypothesis that the CAR-mediated MOA for liver tumorigenesis is of low carcinogenic risk for humans. In this research, in addition to cultured human hepatocyte studies, the usefulness of the humanized chimeric liver mouse models was clearly demonstrated. These data substantially influenced decisions in regulatory toxicology. In this review I comprehensively discuss the human relevance of the CAR-mediated MOA for rodent liver tumorigenesis based on published information, including our recent molecular research on CAR-mediated MOA.
中文翻译:

根据作用方式评估拟除虫菊酯/菊酯诱导的啮齿类动物肝脏肿瘤与人类的相关性的案例†
啮齿类动物的致癌性研究可用于筛查人类致癌物,但并非十全十美。某些作用方式(MOA)会在实验性啮齿动物和人类中均导致癌症,而另一些导致啮齿动物中的癌症却不会在人类中导致癌症。因此,分析通过化学物质在啮齿动物中产生肿瘤的MOA并确定此类肿瘤数据与人类风险的相关性至关重要。最近,获得了实验数据,作为基于MOA评估拟除虫菊酯(美氟菊酯和母氟辛菊酯)和除虫菊酯诱导的大鼠肝肿瘤与人类相关性的案例。根据国际化学安全计划(IPCS)框架进行的MOA分析得出的结论是,实验数据有力地支持了假定的MOA用于甲氟氰菊酯,莫氟氟丁菊酯和除虫菊酯生产的大鼠肝细胞肿瘤是由组成型雄激素受体(CAR)激活介导的。由于甲氟氰菊酯和甲氟氰菊酯是紧密的结构类似物,因此这两种化学药品的可重现结果都对MOA结果充满信心。此外,培养的人类肝细胞研究和人源化嵌合小鼠肝脏研究表明,人类肝细胞(对这些化合物的促有丝分裂作用难以抵抗)和大鼠肝细胞(对它们的促有丝分裂作用敏感)之间存在物种差异。这些数据强烈支持以下假设:CAR介导的MOA用于肝肿瘤发生对人类的致癌风险低。在这项研究中,除了培养的人类肝细胞研究外,还清楚地证明了人源化嵌合肝小鼠模型的有用性。这些数据极大地影响了调节毒理学的决策。在这篇综述中,我基于公开的信息,包括我们最近对CAR介导的MOA的分子研究,全面讨论了CAR介导的MOA与啮齿类动物肝肿瘤发生的人类相关性。
更新日期:2018-01-16
中文翻译:

根据作用方式评估拟除虫菊酯/菊酯诱导的啮齿类动物肝脏肿瘤与人类的相关性的案例†
啮齿类动物的致癌性研究可用于筛查人类致癌物,但并非十全十美。某些作用方式(MOA)会在实验性啮齿动物和人类中均导致癌症,而另一些导致啮齿动物中的癌症却不会在人类中导致癌症。因此,分析通过化学物质在啮齿动物中产生肿瘤的MOA并确定此类肿瘤数据与人类风险的相关性至关重要。最近,获得了实验数据,作为基于MOA评估拟除虫菊酯(美氟菊酯和母氟辛菊酯)和除虫菊酯诱导的大鼠肝肿瘤与人类相关性的案例。根据国际化学安全计划(IPCS)框架进行的MOA分析得出的结论是,实验数据有力地支持了假定的MOA用于甲氟氰菊酯,莫氟氟丁菊酯和除虫菊酯生产的大鼠肝细胞肿瘤是由组成型雄激素受体(CAR)激活介导的。由于甲氟氰菊酯和甲氟氰菊酯是紧密的结构类似物,因此这两种化学药品的可重现结果都对MOA结果充满信心。此外,培养的人类肝细胞研究和人源化嵌合小鼠肝脏研究表明,人类肝细胞(对这些化合物的促有丝分裂作用难以抵抗)和大鼠肝细胞(对它们的促有丝分裂作用敏感)之间存在物种差异。这些数据强烈支持以下假设:CAR介导的MOA用于肝肿瘤发生对人类的致癌风险低。在这项研究中,除了培养的人类肝细胞研究外,还清楚地证明了人源化嵌合肝小鼠模型的有用性。这些数据极大地影响了调节毒理学的决策。在这篇综述中,我基于公开的信息,包括我们最近对CAR介导的MOA的分子研究,全面讨论了CAR介导的MOA与啮齿类动物肝肿瘤发生的人类相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号