当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective high adsorption capacity for Congo red dye of a new 3D supramolecular complex and its magnetic hybrid†
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1039/c7qi00728k Azizolla Beheshti 1, 2, 3, 4, 5 , Faezeh Hashemi 1, 2, 3, 4, 5 , Fatemeh Behvandi 1, 2, 3, 4, 5 , Peter Mayer 6, 7, 8, 9 , Davide Atzei 10, 11, 12, 13
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1039/c7qi00728k Azizolla Beheshti 1, 2, 3, 4, 5 , Faezeh Hashemi 1, 2, 3, 4, 5 , Fatemeh Behvandi 1, 2, 3, 4, 5 , Peter Mayer 6, 7, 8, 9 , Davide Atzei 10, 11, 12, 13
Affiliation
|
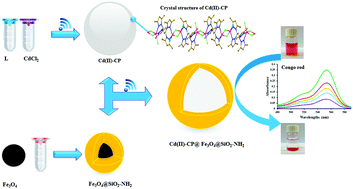
A new non-covalently bonded 3D supramolecular architecture, namely, [CdII2Cl2(μ-Cl)2(μ-L)]n [where L = 1,1,3,3-tetrakis(3,5-dimethyl-1-pyrazolyl)propane] was synthesized and structurally characterized. In this structure, the N4-coordinated pyrazole-based ligand displayed a chelating–bridging mode and bounds to two neighboring CdII centers to create a 1D sinusoidal chain along the b-axis. Each of the cadmium(II) sites adopted a distorted square pyramidal arrangement with a CdN2Cl3 core. The chains were further stabilized by non-covalent bonds such as C–H⋯Cl and C–H⋯π to generate a 3D network. This structure has the ability to incorporate Fe3O4@SiO2-NH2 nanoparticles and build up an Fe3O4@SiO2-NH2@Cd(II)-CP hybrid using a sonochemical method. The bonding established between the Cd(II)-CP and amino-functionalized Fe3O4 nanoparticles improved the separation of the CP by magnetic decantation. The products exhibited a high capacity and high selectivity to adsorb Congo red (CR) from an aqueous solution. The sorption kinetics and equilibrium isotherm were studied in detail using four kinetics and three isotherm models. According to the results that were obtained, the mechanism of the sorption process followed the pseudo-second-order kinetic and Langmuir isotherm models. In general, the magnetic hybrid under consideration can be proposed as a reversible catalyst for the selective adsorption of CR with facile separation from the environment using a magnetic bar.
中文翻译:

新型3D超分子复合物及其磁性杂化物对刚果红染料的选择性高吸附能力†
一种新的非共价键结合的3D超分子体系结构,即[Cd II 2 Cl 2(μ-Cl)2(μ-L)] n [其中L = 1,1,3,3-四(3,5-二甲基)合成了-1-吡唑基丙烷]并进行了结构表征。在这种结构中,N4配位的吡唑基配体表现出螯合-桥接模式,并与两个相邻的Cd II中心结合,沿b轴形成一维正弦链。每个镉(II)位点都采用CdN 2 Cl 3扭曲的方形金字塔形排列核。链通过非共价键(例如C–H⋯Cl和C–H⋯π)进一步稳定,以生成3D网络。该结构具有掺入Fe 3 O 4 @SiO 2 -NH 2纳米颗粒并使用声化学方法建立Fe 3 O 4 @SiO 2 -NH 2 @Cd(II)-CP杂化物的能力。Cd(II)-CP与氨基官能化的Fe 3 O 4之间建立的键合纳米颗粒通过磁倾析改善了CP的分离。该产品具有从水溶液中吸附刚果红(CR)的高容量和高选择性。使用四个动力学模型和三个等温模型详细研究了吸附动力学和平衡等温线。根据获得的结果,吸附过程的机理遵循拟二级动力学和Langmuir等温模型。通常,可以考虑将所考虑的磁性杂化体用作可逆催化剂,以利用磁棒与环境选择性分离来选择性地吸附CR。
更新日期:2018-01-16
中文翻译:

新型3D超分子复合物及其磁性杂化物对刚果红染料的选择性高吸附能力†
一种新的非共价键结合的3D超分子体系结构,即[Cd II 2 Cl 2(μ-Cl)2(μ-L)] n [其中L = 1,1,3,3-四(3,5-二甲基)合成了-1-吡唑基丙烷]并进行了结构表征。在这种结构中,N4配位的吡唑基配体表现出螯合-桥接模式,并与两个相邻的Cd II中心结合,沿b轴形成一维正弦链。每个镉(II)位点都采用CdN 2 Cl 3扭曲的方形金字塔形排列核。链通过非共价键(例如C–H⋯Cl和C–H⋯π)进一步稳定,以生成3D网络。该结构具有掺入Fe 3 O 4 @SiO 2 -NH 2纳米颗粒并使用声化学方法建立Fe 3 O 4 @SiO 2 -NH 2 @Cd(II)-CP杂化物的能力。Cd(II)-CP与氨基官能化的Fe 3 O 4之间建立的键合纳米颗粒通过磁倾析改善了CP的分离。该产品具有从水溶液中吸附刚果红(CR)的高容量和高选择性。使用四个动力学模型和三个等温模型详细研究了吸附动力学和平衡等温线。根据获得的结果,吸附过程的机理遵循拟二级动力学和Langmuir等温模型。通常,可以考虑将所考虑的磁性杂化体用作可逆催化剂,以利用磁棒与环境选择性分离来选择性地吸附CR。











































 京公网安备 11010802027423号
京公网安备 11010802027423号