当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NH2OH–Mediated Lignin Conversion to Isoxazole and Nitrile
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acssuschemeng.7b04114 Hongji Li 1, 2 , Min Wang 1 , Huifang Liu 1, 2 , Nengchao Luo 1, 2 , Jianmin Lu 1 , Chaofeng Zhang 1 , Feng Wang 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acssuschemeng.7b04114 Hongji Li 1, 2 , Min Wang 1 , Huifang Liu 1, 2 , Nengchao Luo 1, 2 , Jianmin Lu 1 , Chaofeng Zhang 1 , Feng Wang 1
Affiliation
|
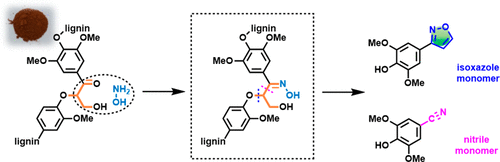
Conversion of lignin to aromatic compounds via C–C/C–O bond cleavage has been an attractive but challenging subject in recent years. We herein report the first protocol that converts lignin models and preoxidized lignin to isoxazole and aromatic nitrile. The isoxazole motif is constructed by condensation of β-hydroxyl ketone with hydroxylamine. Magnesium oxide promotes an oximation reaction and an intramolecular condensation. Aromatic nitriles and esters are obtained via Beckmann rearrangement or an acidolysis reaction depending on the selected additive. The hydroxylamine-mediated strategy works well for the preoxidized lignin conversion to aromatic isoxazole, nitrile, and ester monomers with up to 7.6% yield.
中文翻译:

NH 2 OH介导的木质素转化为异恶唑和腈
近年来,通过C–C / C–O键裂解将木质素转化为芳香族化合物一直是一个有吸引力但具有挑战性的课题。我们在此报告了将木质素模型和预氧化的木质素转化为异恶唑和芳香腈的第一个协议。异恶唑基序是由β-羟基酮与羟胺缩合而成的。氧化镁促进肟化反应和分子内缩合。取决于所选择的添加剂,通过贝克曼重排或酸解反应获得芳族腈和酯。羟胺介导的策略对于将预氧化的木质素转化为芳族异恶唑,腈和酯单体的效果很好,产率高达7.6%。
更新日期:2018-01-16
中文翻译:

NH 2 OH介导的木质素转化为异恶唑和腈
近年来,通过C–C / C–O键裂解将木质素转化为芳香族化合物一直是一个有吸引力但具有挑战性的课题。我们在此报告了将木质素模型和预氧化的木质素转化为异恶唑和芳香腈的第一个协议。异恶唑基序是由β-羟基酮与羟胺缩合而成的。氧化镁促进肟化反应和分子内缩合。取决于所选择的添加剂,通过贝克曼重排或酸解反应获得芳族腈和酯。羟胺介导的策略对于将预氧化的木质素转化为芳族异恶唑,腈和酯单体的效果很好,产率高达7.6%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号