当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐pot Synthesis of Alkynylated Coumarins via Rhodium‐Catalyzed Annulation of Aryl Thiocarbamates with 1,3‐Diynes or Terminal Alkynes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-02-02 , DOI: 10.1002/adsc.201701388 Yuan Gao 1 , Fenfen Zeng 1 , Xudong Sun 1 , Minfeng Zeng 1 , Zhen Yang 1 , Xianqiang Huang 2 , Guodong Shen 2 , Yongsheng Tan 3 , Ruokun Feng 1 , Chenze Qi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-02-02 , DOI: 10.1002/adsc.201701388 Yuan Gao 1 , Fenfen Zeng 1 , Xudong Sun 1 , Minfeng Zeng 1 , Zhen Yang 1 , Xianqiang Huang 2 , Guodong Shen 2 , Yongsheng Tan 3 , Ruokun Feng 1 , Chenze Qi 1
Affiliation
|
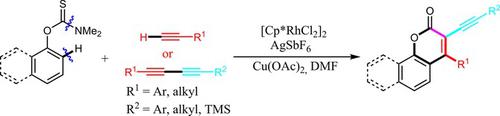
A convenient and selective synthesis of alkynylated coumarins from various aryl thiocarbamates and 1,3‐diynes or terminal alkynes via rhodium‐catalyzed C−H bond activation has been developed. In this transformation, both symmetrical and asymmetrical 1,3‐diynes could be applicable, obtaining various 3‐alkynylated coumarins in moderate to excellent yields. When the substituent is aryl group, the resulting compounds were found to exhibit intense fluorescence in the range of 412–443 nm with quantum yield of up to 0.57 in CH2Cl2. Moreover, the internal alkynes were readily converted to 1,2‐dione, olefins, alkanes, and bisheterocycles under certain conditions.
中文翻译:

铑催化的1,3-二炔或末端炔烃芳基硫代氨基甲酸酯的环化反应一锅法合成炔基香豆素
已经开发了通过铑催化的CH键活化从各种芳基硫代氨基甲酸酯和1,3-二炔或末端炔烃中选择性合成炔基香豆素的方法。在这种转化中,对称和不对称的1,3-二炔都可适用,以中等到极好的收率获得了各种3-炔基化的香豆素。当取代基为芳基时,发现所得化合物在412-443 nm范围内表现出强烈的荧光,在CH 2 Cl 2中的量子产率最高为0.57 。此外,在某些条件下,内部炔烃很容易转化为1,2-二酮,烯烃,烷烃和双环杂环。
更新日期:2018-02-02
中文翻译:

铑催化的1,3-二炔或末端炔烃芳基硫代氨基甲酸酯的环化反应一锅法合成炔基香豆素
已经开发了通过铑催化的CH键活化从各种芳基硫代氨基甲酸酯和1,3-二炔或末端炔烃中选择性合成炔基香豆素的方法。在这种转化中,对称和不对称的1,3-二炔都可适用,以中等到极好的收率获得了各种3-炔基化的香豆素。当取代基为芳基时,发现所得化合物在412-443 nm范围内表现出强烈的荧光,在CH 2 Cl 2中的量子产率最高为0.57 。此外,在某些条件下,内部炔烃很容易转化为1,2-二酮,烯烃,烷烃和双环杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号