当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Multifunctional Spirocyclic Azetidines and Their Application in Drug Discovery
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-02-15 , DOI: 10.1002/chem.201800193 Alexander A. Kirichok 1 , Irina O. Shton 1 , Irina M. Pishel 1 , Sergey A. Zozulya 1, 2 , Petro O. Borysko 1 , Vladimir Kubyshkin 3 , Olha A. Zaporozhets 2 , Andrei A. Tolmachev 1 , Pavel K. Mykhailiuk 1, 2
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-02-15 , DOI: 10.1002/chem.201800193 Alexander A. Kirichok 1 , Irina O. Shton 1 , Irina M. Pishel 1 , Sergey A. Zozulya 1, 2 , Petro O. Borysko 1 , Vladimir Kubyshkin 3 , Olha A. Zaporozhets 2 , Andrei A. Tolmachev 1 , Pavel K. Mykhailiuk 1, 2
Affiliation
|
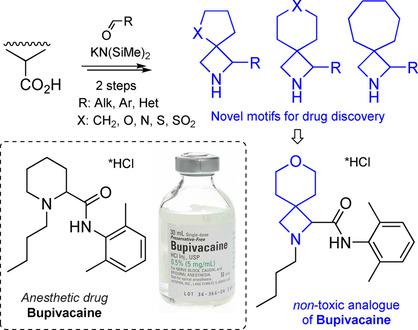
The synthesis of multifunctional spirocycles was achieved from common cyclic carboxylic acids (cyclobutane carboxylate, cyclopentane carboxylate, l‐proline, etc.). The whole sequence included only two chemical steps—synthesis of azetidinones, and reduction into azetidines. The obtained spirocyclic amino acids were incorporated into a structure of the known anesthetic drug Bupivacaine. The obtained analogues were more active and less toxic than the original drug. We believe that this discovery will lead to a wide use of spirocyclic building blocks in drug discovery in the near future.
中文翻译:

多功能螺环氮杂环丁烷的合成及其在药物开发中的应用
多功能螺环的合成从公共环羧酸(环丁烷羧酸,环戊烷羧酸,实现升-脯氨酸等)。整个序列仅包括两个化学步骤-合成氮杂环丁烷酮和还原成氮杂环丁烷。将获得的螺环氨基酸掺入已知麻醉药布比卡因的结构中。所获得的类似物比原始药物具有更高的活性和更低的毒性。我们相信,这一发现将在不久的将来导致螺环构建基团在药物发现中的广泛使用。
更新日期:2018-02-15
中文翻译:

多功能螺环氮杂环丁烷的合成及其在药物开发中的应用
多功能螺环的合成从公共环羧酸(环丁烷羧酸,环戊烷羧酸,实现升-脯氨酸等)。整个序列仅包括两个化学步骤-合成氮杂环丁烷酮和还原成氮杂环丁烷。将获得的螺环氨基酸掺入已知麻醉药布比卡因的结构中。所获得的类似物比原始药物具有更高的活性和更低的毒性。我们相信,这一发现将在不久的将来导致螺环构建基团在药物发现中的广泛使用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号