当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conversion of Furfuryl Alcohol to Levulinic Acid in Aqueous Solution Catalyzed by Shell Thickness-Controlled Arenesulfonic Acid-Functionalized Ethyl-Bridged Organosilica Hollow Nanospheres
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1021/acssuschemeng.7b03133 Sai An 1, 2 , Daiyu Song 1 , Yingnan Sun 1 , Qingqing Zhang 1 , Panpan Zhang 1 , Yihang Guo 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1021/acssuschemeng.7b03133 Sai An 1, 2 , Daiyu Song 1 , Yingnan Sun 1 , Qingqing Zhang 1 , Panpan Zhang 1 , Yihang Guo 1
Affiliation
|
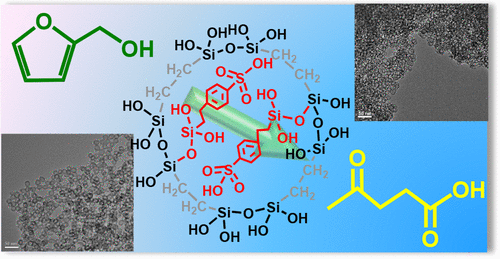
A series of arenesulfonic acid functionalized ethyl-bridged organosilica hollow nanospheres, ArSO3H-Et-HNS, with different shell thicknesses (2–6 nm) were successfully fabricated by P123 and/or F127-directed sol–gel cocondensation route and carefully controlled molar composition of the starting materials in the synthetic gel. The ArSO3H-Et-HNS were applied in the synthesis of levulinic acid (LA) from the hydrolysis of furfuryl alcohol (FAL), and the influence of experimental parameters including solvent nature, volume ratio of FAL-to-solvent-to-water, shell thickness of hollow nanospheres, and reaction temperature were considered. The hydrolysis activity of the ArSO3H-Et-HNS outperformed commercially available HY-zeolite, Amberlyst-15, and p-toluenesulfonic acid, regardless of the shell thickness of the hollow nanospheres; additionally, P123-directed ArSO3H-Et-HNS with the thinnest shell (2 nm) and the largest BET surface area (529 m2 g–1) exhibited the highest hydrolysis activity among various ArSO3H-Et-HNS nanohybrids, and under the conditions of 0.72 mol L–1 FAL in 4:1 acetone-H2O, 3 wt % catalyst, and 120 °C, the yield of LA reached 83.1% after the reaction proceeded for 120 min. The excellent hydrolysis activity of the ArSO3H-Et-HNS was explained in terms of the strong Brønsted acid nature and unique hollow spherical nanostructures with opening shell and hydrophobic surface. Based on the catalytic test results and the identified intermediates, the reaction mechanism of the ArSO3H-Et-HNS-catalyzed hydrolysis of FAL to LA was tentatively revealed. Finally, the reusability of the ArSO3H-Et-HNS was studied through three consecutive catalytic cycles.
中文翻译:

壳厚控制的芳烃磺酸官能化的乙基桥有机硅空心纳米球催化水溶液中糠醇向水溶液中的糠醛酸的转化
通过P123和/或F127定向的溶胶-凝胶共缩聚工艺成功制备了一系列具有不同壳厚度(2–6 nm)的芳烃磺酸官能化的乙基桥连有机硅空心纳米球ArSO 3 H- Et- HNS,并对其进行了精心控制合成凝胶中原料的摩尔组成。将ArSO 3 H- Et -HNS用于糠醇水解(FAL)合成乙酰丙酸(LA),以及实验参数的影响,包括溶剂性质,FAL与溶剂的体积比考虑水,空心纳米球的壳厚度和反应温度。ArSO 3 H- Et的水解活性-HNS的性能优于市售的HY沸石,Amberlyst-15和对甲苯磺酸,而与空心纳米球的壳厚度无关。此外,在最薄的外壳(2 nm)和最大的BET表面积(529 m 2 g –1)的P123指导下的ArSO 3 H- Et -HNS表现出在各种ArSO 3 H- Et -HNS纳米杂交物中最高的水解活性,在4:1丙酮-H 2 O,3 wt%的催化剂和120°C的条件下,在0.72 mol L –1 FAL的条件下,反应进行120分钟后,LA的收率达到83.1%。ArSO 3 H- Et的出色水解活性-HNS用强布朗斯台德酸性质和具有开放壳和疏水表面的独特空心球形纳米结构进行了解释。根据催化测试结果和鉴定出的中间体,初步揭示了ArSO 3 H - Et -HNS催化FAL水解为LA的反应机理。最后,通过三个连续的催化循环研究了ArSO 3 H- Et -HNS的可重用性。
更新日期:2018-01-15
中文翻译:

壳厚控制的芳烃磺酸官能化的乙基桥有机硅空心纳米球催化水溶液中糠醇向水溶液中的糠醛酸的转化
通过P123和/或F127定向的溶胶-凝胶共缩聚工艺成功制备了一系列具有不同壳厚度(2–6 nm)的芳烃磺酸官能化的乙基桥连有机硅空心纳米球ArSO 3 H- Et- HNS,并对其进行了精心控制合成凝胶中原料的摩尔组成。将ArSO 3 H- Et -HNS用于糠醇水解(FAL)合成乙酰丙酸(LA),以及实验参数的影响,包括溶剂性质,FAL与溶剂的体积比考虑水,空心纳米球的壳厚度和反应温度。ArSO 3 H- Et的水解活性-HNS的性能优于市售的HY沸石,Amberlyst-15和对甲苯磺酸,而与空心纳米球的壳厚度无关。此外,在最薄的外壳(2 nm)和最大的BET表面积(529 m 2 g –1)的P123指导下的ArSO 3 H- Et -HNS表现出在各种ArSO 3 H- Et -HNS纳米杂交物中最高的水解活性,在4:1丙酮-H 2 O,3 wt%的催化剂和120°C的条件下,在0.72 mol L –1 FAL的条件下,反应进行120分钟后,LA的收率达到83.1%。ArSO 3 H- Et的出色水解活性-HNS用强布朗斯台德酸性质和具有开放壳和疏水表面的独特空心球形纳米结构进行了解释。根据催化测试结果和鉴定出的中间体,初步揭示了ArSO 3 H - Et -HNS催化FAL水解为LA的反应机理。最后,通过三个连续的催化循环研究了ArSO 3 H- Et -HNS的可重用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号