当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic Tumor–Vascular Model to Study Breast Cancer Cell Invasion and Intravasation
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-01-15 , DOI: 10.1002/adhm.201701257 Supriya Nagaraju 1 , Danh Truong 1 , Ghassan Mouneimne 2 , Mehdi Nikkhah 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-01-15 , DOI: 10.1002/adhm.201701257 Supriya Nagaraju 1 , Danh Truong 1 , Ghassan Mouneimne 2 , Mehdi Nikkhah 1
Affiliation
|
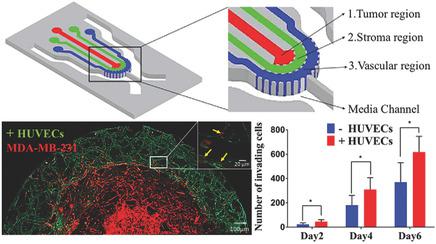
Cancer is a major leading cause of disease‐related death in the world. The severe impact of cancer can be attributed to poor understanding of the mechanisms involved in earliest steps of the metastatic cascade, specifically invasion into the surrounding stroma and intravasation into the blood capillaries. However, conducting integrated biological studies of invasion and intravasation have been challenging, within in vivo models and traditional in vitro assay, due to difficulties in establishing a precise tumor microenvironment. To that end, in this work, a novel 3D microfluidic platform comprised of concentric three‐layer cell‐laden hydrogels for simultaneous investigation of breast cancer cell invasion and intravasation as well as vasculature maturation influenced by tumor–vascular crosstalk is developed. It was demonstrated that the presence of spontaneously formed vasculature enhance MDA‐MB‐231 invasion into the 3D stroma. Following invasion, cancer cells are visualized intravasating into the outer vasculature. Additionally, invading cancer cells significantly reduce vessel diameter while increasing permeability, consistent with previous in vivo studies. Major signaling cytokines involved in tumor–vascular crosstalk that govern cancer cell invasion and intravasation are further identified. Taken together, this platform will enable unique insights of critical biological events within the metastatic cascade, with significant potential for developing efficient cancer therapeutics.
中文翻译:

微流体肿瘤血管模型研究乳腺癌细胞的侵袭和浸润
癌症是世界上与疾病相关的死亡的主要原因。癌症的严重影响可归因于对转移级联的最早步骤所涉及的机制的了解不足,尤其是浸入周围的基质和血管内的血管内。然而,由于难以建立精确的肿瘤微环境,因此在体内模型和传统的体外测定中,进行侵袭和内插的综合生物学研究具有挑战性。为此,在这项工作中,开发了一种新颖的3D微流体平台,该平台由同心的三层载有细胞的水凝胶组成,可同时研究乳腺癌细胞的侵袭和血管内浸入以及受肿瘤与血管间的串扰影响的脉管成熟度。结果表明,自发形成的脉管系统的存在会增强MDA-MB-231对3D基质的侵袭。侵袭后,癌细胞可视化渗入外部脉管系统。另外,与先前的体内研究一致,侵入的癌细胞显着减小了血管直径,同时增加了通透性。进一步确定了与肿瘤-血管串扰有关的主要信号传导细胞因子,这些细胞因子控制着癌细胞的侵袭和血管内侵袭。综上所述,该平台将使转移级联内的关键生物学事件具有独特的见解,并具有开发有效癌症治疗剂的巨大潜力。另外,与先前的体内研究一致,侵入的癌细胞显着减小了血管直径,同时增加了通透性。进一步确定了与肿瘤-血管串扰有关的主要信号传导细胞因子,这些细胞因子控制着癌细胞的侵袭和血管内侵袭。综上所述,该平台将使转移级联内的关键生物学事件具有独特的见解,并具有开发有效癌症治疗剂的巨大潜力。另外,与先前的体内研究一致,侵入的癌细胞显着减小了血管直径,同时增加了通透性。进一步确定了与肿瘤-血管串扰有关的主要信号传导细胞因子,这些细胞因子控制着癌细胞的侵袭和血管内侵袭。综上所述,该平台将使转移级联内的关键生物学事件具有独特的见解,并具有开发有效癌症治疗剂的巨大潜力。
更新日期:2018-01-15
中文翻译:

微流体肿瘤血管模型研究乳腺癌细胞的侵袭和浸润
癌症是世界上与疾病相关的死亡的主要原因。癌症的严重影响可归因于对转移级联的最早步骤所涉及的机制的了解不足,尤其是浸入周围的基质和血管内的血管内。然而,由于难以建立精确的肿瘤微环境,因此在体内模型和传统的体外测定中,进行侵袭和内插的综合生物学研究具有挑战性。为此,在这项工作中,开发了一种新颖的3D微流体平台,该平台由同心的三层载有细胞的水凝胶组成,可同时研究乳腺癌细胞的侵袭和血管内浸入以及受肿瘤与血管间的串扰影响的脉管成熟度。结果表明,自发形成的脉管系统的存在会增强MDA-MB-231对3D基质的侵袭。侵袭后,癌细胞可视化渗入外部脉管系统。另外,与先前的体内研究一致,侵入的癌细胞显着减小了血管直径,同时增加了通透性。进一步确定了与肿瘤-血管串扰有关的主要信号传导细胞因子,这些细胞因子控制着癌细胞的侵袭和血管内侵袭。综上所述,该平台将使转移级联内的关键生物学事件具有独特的见解,并具有开发有效癌症治疗剂的巨大潜力。另外,与先前的体内研究一致,侵入的癌细胞显着减小了血管直径,同时增加了通透性。进一步确定了与肿瘤-血管串扰有关的主要信号传导细胞因子,这些细胞因子控制着癌细胞的侵袭和血管内侵袭。综上所述,该平台将使转移级联内的关键生物学事件具有独特的见解,并具有开发有效癌症治疗剂的巨大潜力。另外,与先前的体内研究一致,侵入的癌细胞显着减小了血管直径,同时增加了通透性。进一步确定了与肿瘤-血管串扰有关的主要信号传导细胞因子,这些细胞因子控制着癌细胞的侵袭和血管内侵袭。综上所述,该平台将使转移级联内的关键生物学事件具有独特的见解,并具有开发有效癌症治疗剂的巨大潜力。







































 京公网安备 11010802027423号
京公网安备 11010802027423号