当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH‐Responsive PEG–Doxorubicin‐Encapsulated Aza‐BODIPY Nanotheranostic Agent for Imaging‐Guided Synergistic Cancer Therapy
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-01-15 , DOI: 10.1002/adhm.201701272 Dapeng Chen 1 , Qianyun Tang 1 , Jianhua Zou 1 , Xiaoyan Yang 1 , Wei Huang 1, 2 , Qi Zhang 3 , Jinjun Shao 1 , Xiaochen Dong 1, 4
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-01-15 , DOI: 10.1002/adhm.201701272 Dapeng Chen 1 , Qianyun Tang 1 , Jianhua Zou 1 , Xiaoyan Yang 1 , Wei Huang 1, 2 , Qi Zhang 3 , Jinjun Shao 1 , Xiaochen Dong 1, 4
Affiliation
|
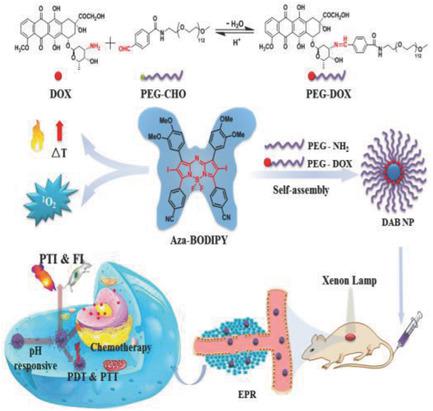
Synergistic cancer therapy is of great interest for multiple advantages, such as excellent targeting accuracy, low side effects, and enhanced therapeutic efficiency. Herein, a near‐infrared photosensitizer aza‐BODIPY (AB) with high singlet oxygen quantum yield (ΦΔ = 82%) is designed and synthesized. With Schiff's base obtained from condensation reaction between doxorubicin (DOX) and polyethylene glycol‐benzaldehyde (PEG–CHO) as the polymer matrix, aza‐BODIPY is encapsulated to afford hydrophilic nanoparticles (DAB NPs). The DAB NPs exhibit high reactive oxygen species (ROS) generation rate and outstanding photothermal conversion efficiency (η = 38.3%) under irradiation. In vivo fluorescence‐ and photothermal‐imaging (PTI) results demonstrate that DAB NPs can specifically accumulate at tumor sites and serve as dual‐modal imaging probe for cancer diagnosis. Particularly, triggered by acidic tumor microenvironment, the HCN bond of Schiff's base would be broken simultaneously, resulting in the efficient release of DOX from DAB NPs at tumor sites as well as enhancing the targeting performance of chemotherapeutics. Compared with free DOX and aza‐BODIPY nanoparticles, DAB NPs can inhibit tumor growth more effectively through pH‐responsive photodynamic/photothermal/chemo synergistic therapy. This report may also present a practicable strategy to develop a pH‐responsive nanotheranostic agent for tumor targeting, imaging, and therapy.
中文翻译:

pH响应的PEG-阿霉素包封的Aza-BODIPY纳米热疗剂,用于影像引导的协同癌症治疗。
协同癌症治疗因多种优势而备受关注,例如出色的靶向准确性,较低的副作用和增强的治疗效率。这里,近红外光敏剂氮杂BODIPY(AB)具有高的单线态氧量子产率(Φ Δ= 82%)进行了设计和合成。通过将阿霉素(DOX)与聚乙二醇-苯甲醛(PEG-CHO)之间的缩合反应获得的席夫碱作为聚合物基质,将aza-BODIPY封装起来,制成亲水性纳米颗粒(DAB NPs)。DAB NPs在辐照下表现出高活性氧(ROS)生成速率和出色的光热转化效率(η= 38.3%)。体内荧光和光热成像(PTI)结果表明,DAB NP可以特异性地聚集在肿瘤部位,并且可以用作癌症诊断的双峰成像探针。特别是,通过酸性肿瘤微环境中,触发 HCN Schiff碱的键会同时被破坏,从而导致DAX NPs在肿瘤部位有效释放DOX,并增强化学疗法的靶向性能。与游离DOX和aza-BODIPY纳米粒子相比,DAB NPs可通过pH响应光动力/光热/化学协同疗法更有效地抑制肿瘤生长。该报告也可能提出一种可行的策略,以开发用于肿瘤靶向,成像和治疗的pH响应纳米热敏剂。
更新日期:2018-01-15
中文翻译:

pH响应的PEG-阿霉素包封的Aza-BODIPY纳米热疗剂,用于影像引导的协同癌症治疗。
协同癌症治疗因多种优势而备受关注,例如出色的靶向准确性,较低的副作用和增强的治疗效率。这里,近红外光敏剂氮杂BODIPY(AB)具有高的单线态氧量子产率(Φ Δ= 82%)进行了设计和合成。通过将阿霉素(DOX)与聚乙二醇-苯甲醛(PEG-CHO)之间的缩合反应获得的席夫碱作为聚合物基质,将aza-BODIPY封装起来,制成亲水性纳米颗粒(DAB NPs)。DAB NPs在辐照下表现出高活性氧(ROS)生成速率和出色的光热转化效率(η= 38.3%)。体内荧光和光热成像(PTI)结果表明,DAB NP可以特异性地聚集在肿瘤部位,并且可以用作癌症诊断的双峰成像探针。特别是,通过酸性肿瘤微环境中,触发 HCN Schiff碱的键会同时被破坏,从而导致DAX NPs在肿瘤部位有效释放DOX,并增强化学疗法的靶向性能。与游离DOX和aza-BODIPY纳米粒子相比,DAB NPs可通过pH响应光动力/光热/化学协同疗法更有效地抑制肿瘤生长。该报告也可能提出一种可行的策略,以开发用于肿瘤靶向,成像和治疗的pH响应纳米热敏剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号