当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A high-throughput method for orthophosphate determination of thermostable membrane-bound pyrophosphatase activity
Analytical Methods ( IF 2.7 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1039/c7ay02558k Keni Vidilaseris 1, 2, 3, 4, 5 , Juho Kellosalo 3, 5, 6 , Adrian Goldman 1, 2, 3, 4, 5
Analytical Methods ( IF 2.7 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1039/c7ay02558k Keni Vidilaseris 1, 2, 3, 4, 5 , Juho Kellosalo 3, 5, 6 , Adrian Goldman 1, 2, 3, 4, 5
Affiliation
|
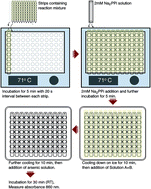
Membrane-bound pyrophosphatases (mPPases) are homodimeric integral membrane proteins that hydrolyse pyrophosphate into orthophosphates coupled to the active transport of protons or sodium ions across membranes. They occur in bacteria, archaea, plants, and protist parasites. As they are essential in protist parasites and there are no homologous proteins in animals and humans, these enzymes represent an excellent drug target for treating protistal diseases. Experimental screening to find drug candidates is an important step to discover new hit compounds. For that, a cheap, simple, and robust assay is needed. Here we report the application of the molybdenum blue reaction method for a medium throughput microplate activity assay of the hyperthermophilic bacterium Thermotoga maritima mPPase and the possible application of the assay to screen inhibitors of membrane-bound pyrophosphatases.
中文翻译:

高通量正磷酸盐测定热稳定膜结合焦磷酸酶活性的方法
膜结合的焦磷酸酶(mPPase)是同二聚体的整体膜蛋白,可将焦磷酸水解为正磷酸,与质子或钠离子跨膜的主动转运相结合。它们发生在细菌,古细菌,植物和原生质寄生虫中。由于它们在原生动物寄生虫中是必不可少的,并且在动物和人类中没有同源蛋白质,因此这些酶代表了治疗前列腺炎的极好的药物靶标。实验筛选以寻找候选药物是发现新的流行化合物的重要步骤。为此,需要一种便宜,简单且耐用的测定方法。在这里,我们报道了钼蓝反应方法在中温微孔板嗜热菌马氏菌的中等通量微孔板活性分析中的应用。 mPPase及其在筛选膜结合焦磷酸酶抑制剂中的可能应用。
更新日期:2018-01-15
中文翻译:

高通量正磷酸盐测定热稳定膜结合焦磷酸酶活性的方法
膜结合的焦磷酸酶(mPPase)是同二聚体的整体膜蛋白,可将焦磷酸水解为正磷酸,与质子或钠离子跨膜的主动转运相结合。它们发生在细菌,古细菌,植物和原生质寄生虫中。由于它们在原生动物寄生虫中是必不可少的,并且在动物和人类中没有同源蛋白质,因此这些酶代表了治疗前列腺炎的极好的药物靶标。实验筛选以寻找候选药物是发现新的流行化合物的重要步骤。为此,需要一种便宜,简单且耐用的测定方法。在这里,我们报道了钼蓝反应方法在中温微孔板嗜热菌马氏菌的中等通量微孔板活性分析中的应用。 mPPase及其在筛选膜结合焦磷酸酶抑制剂中的可能应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号