当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Reaction Mechanism of Na–O2 Batteries using Environmental Transmission Electron Microscopy
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acsenergylett.7b01273 Won-Jin Kwak 1 , Langli Luo 2 , Hun-Gi Jung 3 , Chongmin Wang 2 , Yang-Kook Sun 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acsenergylett.7b01273 Won-Jin Kwak 1 , Langli Luo 2 , Hun-Gi Jung 3 , Chongmin Wang 2 , Yang-Kook Sun 1
Affiliation
|
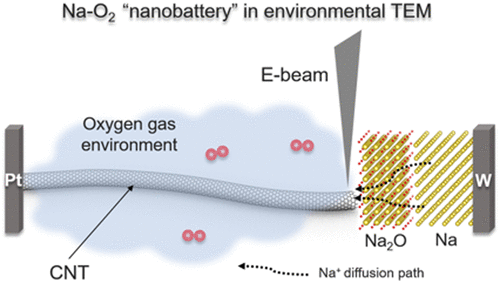
Sodium–oxygen (Na–O2) batteries are being extensively studied because of their higher energy efficiency compared to that of lithium oxygen (Li–O2) batteries. The critical challenges in the development of Na–O2 batteries include the elucidation of the reaction mechanism, reaction products, and the structural and chemical evolution of the reaction products and their correlation with battery performance. For the first time, in situ transmission electron microscopy was employed to probe the reaction mechanism and structural evolution of the discharge products in Na–O2 batteries. The discharge product was featured by the formation of both cubic and conformal NaO2. It was noticed that the impingement of the reaction product (NaO2) led to particle coarsening through coalescence. We investigated the stability of the discharge product and observed that the reaction product NaO2 was stable in the case of the solid electrolyte. The present work provides unprecedented insight into the development of Na–O2 batteries.
中文翻译:

使用环境透射电子显微镜揭示Na–O 2电池的反应机理
钠氧(Na-O 2)电池比锂氧(Li-O 2)电池具有更高的能效,因此正在得到广泛的研究。Na–O 2电池开发中的关键挑战包括阐明反应机理,反应产物以及反应产物的结构和化学演化及其与电池性能的关系。首次采用原位透射电子显微镜探测Na–O 2电池的反应机理和放电产物的结构演变。放电产物的特征是同时形成立方和保形的NaO 2。注意到反应产物(NaO 2)的撞击导致通过聚结使颗粒粗化。我们研究了放电产物的稳定性,并观察到在固体电解质的情况下反应产物NaO 2是稳定的。目前的工作为Na-O 2电池的发展提供了空前的见识。
更新日期:2018-01-17
中文翻译:

使用环境透射电子显微镜揭示Na–O 2电池的反应机理
钠氧(Na-O 2)电池比锂氧(Li-O 2)电池具有更高的能效,因此正在得到广泛的研究。Na–O 2电池开发中的关键挑战包括阐明反应机理,反应产物以及反应产物的结构和化学演化及其与电池性能的关系。首次采用原位透射电子显微镜探测Na–O 2电池的反应机理和放电产物的结构演变。放电产物的特征是同时形成立方和保形的NaO 2。注意到反应产物(NaO 2)的撞击导致通过聚结使颗粒粗化。我们研究了放电产物的稳定性,并观察到在固体电解质的情况下反应产物NaO 2是稳定的。目前的工作为Na-O 2电池的发展提供了空前的见识。









































 京公网安备 11010802027423号
京公网安备 11010802027423号