当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Azasilicon-bridged heterocyclic arylamines: syntheses, structures and photophysical properties†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1039/c7nj03876c Shifang Yuan 1, 2, 3, 4, 5 , Lijing Wang 2, 3, 4, 5 , Chuanbing Huang 4, 6, 7, 8 , Chunxia Niu 2, 3, 4, 5, 6 , Kai Xiang 4, 6, 7, 8 , Caihong Xu 4, 6, 7, 8 , Gregory A. Solan 4, 6, 7, 8, 9 , Hongwei Ma 4, 10, 11, 12 , Wen-Hua Sun 4, 6, 7, 8
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1039/c7nj03876c Shifang Yuan 1, 2, 3, 4, 5 , Lijing Wang 2, 3, 4, 5 , Chuanbing Huang 4, 6, 7, 8 , Chunxia Niu 2, 3, 4, 5, 6 , Kai Xiang 4, 6, 7, 8 , Caihong Xu 4, 6, 7, 8 , Gregory A. Solan 4, 6, 7, 8, 9 , Hongwei Ma 4, 10, 11, 12 , Wen-Hua Sun 4, 6, 7, 8
Affiliation
|
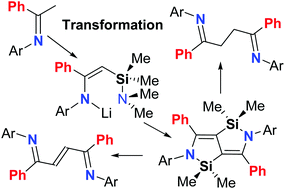
The lithium κ1-enamides, Me2NSiMe2CHC(Ph)-{2,6-(R1)2-4-(R2)C6H2}NLi·3THF (R1 = iPr, R2 = H L1; R1 = Et, R2 = H L2; R1 = Me, R2 = H L3; R1 = R2 = Me L4; R1 = Et, R2 = Me L5), in the presence of titanium tetrachloride, undergo intermolecular rearrangement cyclization reactions resulting in 1,3-migration of the silicon groups and the elimination of dimethylamine affording five examples of bis-azasilicon-bridged heterocyclic arylamines, [{2,6-(R1)2-4-(R2)C6H2}N(Ph)CCSiMe2]2 (R1 = iPr, R2 = H D1; R1 = Et, R2 = H D2; R1 = Me, R2 = H D3; R1 = R2 = Me D4; R1 = Et, R2 = Me D5) in good yield, respectively. The molecular structures of D1–D5 show the two fused N–Si–C–C–C rings to be co-planar indicative of extended π-conjugation, while their photophysical properties reveal them to be green/blue emitting with high luminescence quantum yields (ΦF range: 75–99%). Furthermore, the compounds D serve as versatile reactants undergoing ring opening on hydrolysis to afford the saturated 1,4-diimines [{2,6-(R1)2-4-(R2)C6H2}N(Ph)C-CH2]2 (R1 = iPr, R2 = H E1; R1 = Et, R2 = H E2; R1 = Me, R2 = H E3; R1 = R2 = Me E4; R1 = Et, R2 = Me E5). Alternatively, D can be employed in a redox-promoted cascade reaction to afford the conjugated 1,4-diimines, (E)-[{2,6-(R1)2-4-(R2)C6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)CH]2 (R1 = iPr, R2 = H F1; R1 = Et, R2 = H F2; R1 = Me, R2 = H F3; R1 = R2 = Me F4; R1 = Et, R2 = Me F5). In addition to D1–D5, E1–E3, E5, F2 and F3 have been the subject of single crystal X-ray diffraction studies.
C(Ph)CH]2 (R1 = iPr, R2 = H F1; R1 = Et, R2 = H F2; R1 = Me, R2 = H F3; R1 = R2 = Me F4; R1 = Et, R2 = Me F5). In addition to D1–D5, E1–E3, E5, F2 and F3 have been the subject of single crystal X-ray diffraction studies.
中文翻译:

氮杂硅桥连的杂环芳基胺:合成,结构和光物理性质†
锂κ 1个-enamides中,Me 2 NSiMe 2 CHC(PH) - {2,6-(R 1)2 -4-(R 2)C 6 H ^ 2 } NLi等·3THF(R 1 =我镨,R 2 = H L1 ; R 1 = Et,R 2 = H L2 ; R 1 = Me,R 2 = H L3 ; R 1 = R 2 = Me L4 ; R 1 = Et,R 2 = Me L5),在四氯化钛存在下,进行分子间重排环化反应,导致硅基团进行1,3迁移并消除二甲基胺,从而得到双氮杂硅桥连的杂环芳基胺的五个例子,[{2,6-(R 1)2 -4-(R 2)C 6 H 2 } N(Ph)CCSiMe 2 ] 2(R 1 = i Pr,R 2 = H D1 ; R 1 = Et,R 2 = H D2 ; R 1 = Me,R 2 = H D3; R 1 = R2 =我D4 ; R 1= Et,R 2= Me D5)分别具有良好的产率。D1-D5的分子结构显示两个稠合的N-Si- CC-CC环是共面的,表示扩展了π共轭,而它们的光物理性质表明它们是绿色/蓝色发射的,具有高发光量子产率( Φ ˚F范围:75-99%)。此外,化合物D用作通用反应物,其在水解时经历开环反应,得到饱和的1,4-二亚胺[{2,6-(R 1) 2 -4-(R 2)C 6 H 2 } N(Ph) C-CH 2] 2(R 1 = i Pr,R 2 = H E1; R 1 = Et,R 2 = H E2; R 1 = Me,R 2 = H E3; R 1 = R 2 = Me E4; R 1 = Et ,R 2 = Me E5)。或者,可以将D用于氧化还原促进的级联反应中,以得到共轭的1,4-二亚胺,(E)-[{2,6-(R 1)2 -4-(R 2)C 6 H2 } N![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)CH] 2(R 1 = i Pr,R 2 = H F1 ; R 1 = Et,R 2 = H F2 ; R 1 = Me,R 2 = H F3 ; R 1 = R 2= Me F4; R 1= Et,R 2= Me F5)。除D1-D5外, E1-E3, E5, F2和F3也是单晶X射线衍射研究的主题。
C(Ph)CH] 2(R 1 = i Pr,R 2 = H F1 ; R 1 = Et,R 2 = H F2 ; R 1 = Me,R 2 = H F3 ; R 1 = R 2= Me F4; R 1= Et,R 2= Me F5)。除D1-D5外, E1-E3, E5, F2和F3也是单晶X射线衍射研究的主题。
更新日期:2018-01-15
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)CH]2 (R1 = iPr, R2 = H F1; R1 = Et, R2 = H F2; R1 = Me, R2 = H F3; R1 = R2 = Me F4; R1 = Et, R2 = Me F5). In addition to D1–D5, E1–E3, E5, F2 and F3 have been the subject of single crystal X-ray diffraction studies.
C(Ph)CH]2 (R1 = iPr, R2 = H F1; R1 = Et, R2 = H F2; R1 = Me, R2 = H F3; R1 = R2 = Me F4; R1 = Et, R2 = Me F5). In addition to D1–D5, E1–E3, E5, F2 and F3 have been the subject of single crystal X-ray diffraction studies.
中文翻译:

氮杂硅桥连的杂环芳基胺:合成,结构和光物理性质†
锂κ 1个-enamides中,Me 2 NSiMe 2 CHC(PH) - {2,6-(R 1)2 -4-(R 2)C 6 H ^ 2 } NLi等·3THF(R 1 =我镨,R 2 = H L1 ; R 1 = Et,R 2 = H L2 ; R 1 = Me,R 2 = H L3 ; R 1 = R 2 = Me L4 ; R 1 = Et,R 2 = Me L5),在四氯化钛存在下,进行分子间重排环化反应,导致硅基团进行1,3迁移并消除二甲基胺,从而得到双氮杂硅桥连的杂环芳基胺的五个例子,[{2,6-(R 1)2 -4-(R 2)C 6 H 2 } N(Ph)CCSiMe 2 ] 2(R 1 = i Pr,R 2 = H D1 ; R 1 = Et,R 2 = H D2 ; R 1 = Me,R 2 = H D3; R 1 = R2 =我D4 ; R 1= Et,R 2= Me D5)分别具有良好的产率。D1-D5的分子结构显示两个稠合的N-Si- CC-CC环是共面的,表示扩展了π共轭,而它们的光物理性质表明它们是绿色/蓝色发射的,具有高发光量子产率( Φ ˚F范围:75-99%)。此外,化合物D用作通用反应物,其在水解时经历开环反应,得到饱和的1,4-二亚胺[{2,6-(R 1) 2 -4-(R 2)C 6 H 2 } N(Ph) C-CH 2] 2(R 1 = i Pr,R 2 = H E1; R 1 = Et,R 2 = H E2; R 1 = Me,R 2 = H E3; R 1 = R 2 = Me E4; R 1 = Et ,R 2 = Me E5)。或者,可以将D用于氧化还原促进的级联反应中,以得到共轭的1,4-二亚胺,(E)-[{2,6-(R 1)2 -4-(R 2)C 6 H2 } N
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)CH] 2(R 1 = i Pr,R 2 = H F1 ; R 1 = Et,R 2 = H F2 ; R 1 = Me,R 2 = H F3 ; R 1 = R 2= Me F4; R 1= Et,R 2= Me F5)。除D1-D5外, E1-E3, E5, F2和F3也是单晶X射线衍射研究的主题。
C(Ph)CH] 2(R 1 = i Pr,R 2 = H F1 ; R 1 = Et,R 2 = H F2 ; R 1 = Me,R 2 = H F3 ; R 1 = R 2= Me F4; R 1= Et,R 2= Me F5)。除D1-D5外, E1-E3, E5, F2和F3也是单晶X射线衍射研究的主题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号