当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
o-Iodoxybenzoic acid mediated generation of aryl free radicals: synthesis of stilbenes through C–C cross-coupling with β-nitrostyrenes†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1039/c7nj04701k Ganesh Wagh 1, 2, 3, 4, 5 , Snehalata Autade 1, 2, 3, 4, 5 , Pravin C. Patil 1, 2, 3, 4, 5 , Krishnacharya G. Akamanchi 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-01-15 00:00:00 , DOI: 10.1039/c7nj04701k Ganesh Wagh 1, 2, 3, 4, 5 , Snehalata Autade 1, 2, 3, 4, 5 , Pravin C. Patil 1, 2, 3, 4, 5 , Krishnacharya G. Akamanchi 1, 2, 3, 4, 5
Affiliation
|
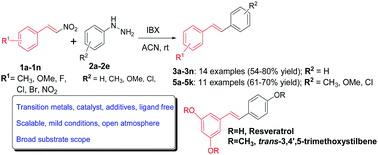
The generation of an aryl free radicals in the presence of nitrostyrenes through a combination of aryl hydrazines and o-iodoxybenzoic acid led to the synthesis of stilbenes by forming a new carbon–carbon bond after subsequent elimination of a nitrosyl radical. The symmetrical and unsymmetrical stilbenes with excellent E-selectivity were synthesized in good yields, with advantages of broad substrate scope and transition metal free, mild reaction conditions, under an open atmosphere in a short reaction time. A free radical mediated mechanism was postulated and supported by radical trapping experiments. Application of the developed methodology is demonstrated through a simple, two step synthesis of resveratrol, a valuable stilbenoid for anticancer and neurological studies via its prodrug E-1,3-dimethoxy-5-(4-methoxystyryl)benzene.
中文翻译:

邻碘氧苯甲酸介导的芳基自由基的生成:通过C–C与β-硝基苯乙烯的交叉偶联来合成芪类化合物†
在硝基苯乙烯存在下,通过芳基肼和邻碘氧苯甲酸的组合生成芳基自由基导致了斯蒂苯类的合成,其方法是在随后消除亚硝酰基基团后形成新的碳-碳键。以高收率合成了具有优异E选择性的对称和不对称对苯二甲酸酯,具有宽的底物范围和无过渡金属,温和的反应条件,在开放的气氛中,较短的反应时间内的优势。自由基介导的机制是由自由基诱捕实验推测和支持的。通过简单的两步合成白藜芦醇(一种对抗癌和神经系统研究有用的有价值的类芪),证明了所开发方法的应用通过其前药E -1,3-二甲氧基-5-(4-甲氧基苯乙烯基)苯。
更新日期:2018-01-15
中文翻译:

邻碘氧苯甲酸介导的芳基自由基的生成:通过C–C与β-硝基苯乙烯的交叉偶联来合成芪类化合物†
在硝基苯乙烯存在下,通过芳基肼和邻碘氧苯甲酸的组合生成芳基自由基导致了斯蒂苯类的合成,其方法是在随后消除亚硝酰基基团后形成新的碳-碳键。以高收率合成了具有优异E选择性的对称和不对称对苯二甲酸酯,具有宽的底物范围和无过渡金属,温和的反应条件,在开放的气氛中,较短的反应时间内的优势。自由基介导的机制是由自由基诱捕实验推测和支持的。通过简单的两步合成白藜芦醇(一种对抗癌和神经系统研究有用的有价值的类芪),证明了所开发方法的应用通过其前药E -1,3-二甲氧基-5-(4-甲氧基苯乙烯基)苯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号