当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Graphene‐Tailored Thermodynamics and Kinetics to Fabricate Metal Borohydride Nanoparticles with High Purity and Enhanced Reversibility
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-01-15 , DOI: 10.1002/aenm.201702975 Hongyu Zhang 1 , Guanglin Xia 2 , Jian Zhang 3 , Dalin Sun 1, 4 , Zaiping Guo 2 , Xuebin Yu 1, 4
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-01-15 , DOI: 10.1002/aenm.201702975 Hongyu Zhang 1 , Guanglin Xia 2 , Jian Zhang 3 , Dalin Sun 1, 4 , Zaiping Guo 2 , Xuebin Yu 1, 4
Affiliation
|
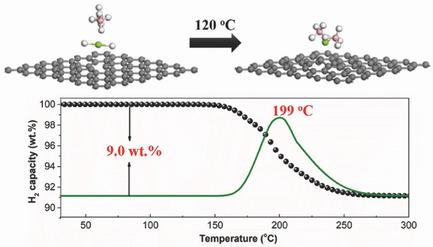
Due to their ultrahigh theoretical capacity, metal borohydrides are considered to be one of the most promising candidate hydrogen storage materials. Their application still suffers, however, from high operating temperature, sluggish kinetics, and poor reversibility. Designing nanostructures is an effective way of addressing these issues, but seeking suitable approaches remains a big challenge. Here, a space‐confined solid‐gas reaction to synthesize Mg(BH4)2 nanoparticles supported on grapheme is reported, which serves as the structural support for the dispersed Mg(BH4)2 nanoparticles. More notably, density functional theory calculations reveal that graphene could weaken both the MgH bonds of MgH2 and BB bonds of B2H6, which could thermodynamically and kinetically facilitate the chemical transformation to synthesize Mg(BH4)2 with high purity. Because of the synergistic effects of both the significant reduction in particle size and the catalytic effect of graphene, an onset dehydrogenation temperature of ≈154 °C is observed for Mg(BH4)2 nanoparticles, and a complete dehydrogenation could be achieved at a temperature as low as 225 °C, with the formation of MgB2 as the by‐product. This work provides a new perspective to tailoring the thermodynamics and kinetics of chemical reactions toward the favorable synthesis of functional inorganic materials.
中文翻译:

石墨烯定制的热力学和动力学制备高纯度和增强的可逆性金属硼氢化物纳米粒子
由于其超高的理论容量,金属硼氢化物被认为是最有前途的候选储氢材料之一。然而,它们的应用仍然受到操作温度高,动力学迟缓和可逆性差的困扰。设计纳米结构是解决这些问题的有效方法,但是寻求合适的方法仍然是一个巨大的挑战。在这里,报道了一种空间受限的固溶反应以合成支撑在石墨烯上的Mg(BH 4)2纳米颗粒,该反应为分散的Mg(BH 4)2纳米颗粒提供了结构支撑。更值得注意的是,密度泛函理论计算表明,石墨烯可能会削弱两者的镁的MgH的H键B 2 H 6的2和BB键可以热力学和动力学促进化学转化,从而以高纯度合成Mg(BH 4)2。由于显着减小粒径和石墨烯的催化作用的协同作用,Mg(BH 4)2纳米粒子的起始脱氢温度为≈154°C ,并且在一定温度下可以实现完全脱氢最低至225°C,形成MgB 2作为副产品。这项工作为调整化学反应的热力学和动力学方向以实现功能性无机材料的良好合成提供了新的视角。
更新日期:2018-01-15
中文翻译:

石墨烯定制的热力学和动力学制备高纯度和增强的可逆性金属硼氢化物纳米粒子
由于其超高的理论容量,金属硼氢化物被认为是最有前途的候选储氢材料之一。然而,它们的应用仍然受到操作温度高,动力学迟缓和可逆性差的困扰。设计纳米结构是解决这些问题的有效方法,但是寻求合适的方法仍然是一个巨大的挑战。在这里,报道了一种空间受限的固溶反应以合成支撑在石墨烯上的Mg(BH 4)2纳米颗粒,该反应为分散的Mg(BH 4)2纳米颗粒提供了结构支撑。更值得注意的是,密度泛函理论计算表明,石墨烯可能会削弱两者的镁的MgH的H键B 2 H 6的2和BB键可以热力学和动力学促进化学转化,从而以高纯度合成Mg(BH 4)2。由于显着减小粒径和石墨烯的催化作用的协同作用,Mg(BH 4)2纳米粒子的起始脱氢温度为≈154°C ,并且在一定温度下可以实现完全脱氢最低至225°C,形成MgB 2作为副产品。这项工作为调整化学反应的热力学和动力学方向以实现功能性无机材料的良好合成提供了新的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号