当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ shRNA Synthesis on DNA–Polylactide Nanoparticles to Treat Multidrug Resistant Breast Cancer
Advanced Materials ( IF 27.4 ) Pub Date : 2018-01-15 , DOI: 10.1002/adma.201705737 Qianqian Ni 1, 2 , Fuwu Zhang 2 , Yunlei Zhang 1 , Guizhi Zhu 2 , Zhe Wang 2 , Zhaogang Teng 1 , Chunyan Wang 1 , Bryant C. Yung 2 , Gang Niu 2 , Guangming Lu 1 , Longjiang Zhang 1 , Xiaoyuan Chen 2
Advanced Materials ( IF 27.4 ) Pub Date : 2018-01-15 , DOI: 10.1002/adma.201705737 Qianqian Ni 1, 2 , Fuwu Zhang 2 , Yunlei Zhang 1 , Guizhi Zhu 2 , Zhe Wang 2 , Zhaogang Teng 1 , Chunyan Wang 1 , Bryant C. Yung 2 , Gang Niu 2 , Guangming Lu 1 , Longjiang Zhang 1 , Xiaoyuan Chen 2
Affiliation
|
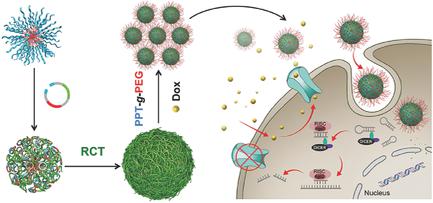
Nanomedicine has shown unprecedented potential for cancer theranostics. Nucleic acid (e.g., DNA and RNA) nanomedicines are of particular interest for combination therapy with chemotherapeutics. However, current nanotechnologies to construct such nucleic acid nanomedicines, which rely on chemical conjugation or physical complexation of nucleic acids with chemotherapeutics, have restrained their clinical translation due to limitations such as low drug loading efficiency and poor biostability. Herein, in situ rolling circle transcription (RCT) is applied to synthesize short hairpin RNA (shRNA) on amphiphilic DNA–polylactide (PLA) micelles. Core–shell PLA@poly‐shRNA structures that codeliver a high payload of doxorubicin (Dox) and multidrug resistance protein 1 (MDR1) targeted shRNA for MDR breast cancer (BC) therapy are developed. DNA–PLA conjugates are first synthesized, which then self‐assemble into amphiphilic DNA–PLA micelles; next, using the conjugated DNA as a promoter, poly‐shRNA is synthesized on DNA–PLA micelles via RCT, generating PLA@poly‐shRNA microflowers; and finally, microflowers are electrostatically condensed into nanoparticles using biocompatible and multifunctional poly(ethylene glycol)‐grafted polypeptides (PPT‐g‐PEG). These PLA@poly‐shRNA@PPT‐g‐PEG nanoparticles are efficiently delivered into MDR breast cancer cells and accumulated in xenograft tumors, leading to MDR1 silencing, intracellular Dox accumulation, potentiated apoptosis, and enhanced tumor therapeutic efficacy. Overall, this nanomedicine platform is promising to codeliver anticancer nucleic acid therapeutics and chemotherapeutics.
中文翻译:

DNA-聚乳酸纳米颗粒的原位shRNA合成治疗多药耐药性乳腺癌
纳米医学已显示出癌症治疗学空前的潜力。核酸(例如DNA和RNA)纳米药物特别适合与化学疗法联合治疗。然而,依赖于诸如药物装载效率低和生物稳定性差的局限性,目前依赖于核酸与化学疗法的化学缀合或物理络合来构建此类核酸纳米药物的纳米技术已经限制了它们的临床翻译。本文中,原位滚动环转录(RCT)用于在两亲性DNA-聚丙交酯(PLA)胶束上合成短发夹RNA(shRNA)。核心-外壳PLA @ poly-shRNA结构可编码提供高有效量的阿霉素(Dox)和多药耐药蛋白1(MDR1开发了用于MDR乳腺癌(BC)治疗的靶向shRNA。首先合成DNA-PLA结合物,然后自组装成两亲性DNA-PLA胶束。接下来,使用结合的DNA作为启动子,通过RCT在DNA-PLA胶束上合成poly-shRNA,产生PLA @ poly-shRNA微型花。最后,使用生物相容的多功能聚乙二醇接枝多肽(PPT- g- PEG)将微花静电凝结成纳米颗粒。这些PLA @ poly-shRNA @ PPT - g - PEG纳米颗粒可以有效地传递到MDR乳腺癌细胞中并积聚在异种移植肿瘤中,从而导致MDR1沉默,细胞内Dox积累,增强的凋亡和增强的肿瘤治疗功效。总体而言,该纳米医学平台有望用于编码交付抗癌核酸疗法和化学疗法。
更新日期:2018-01-15
中文翻译:

DNA-聚乳酸纳米颗粒的原位shRNA合成治疗多药耐药性乳腺癌
纳米医学已显示出癌症治疗学空前的潜力。核酸(例如DNA和RNA)纳米药物特别适合与化学疗法联合治疗。然而,依赖于诸如药物装载效率低和生物稳定性差的局限性,目前依赖于核酸与化学疗法的化学缀合或物理络合来构建此类核酸纳米药物的纳米技术已经限制了它们的临床翻译。本文中,原位滚动环转录(RCT)用于在两亲性DNA-聚丙交酯(PLA)胶束上合成短发夹RNA(shRNA)。核心-外壳PLA @ poly-shRNA结构可编码提供高有效量的阿霉素(Dox)和多药耐药蛋白1(MDR1开发了用于MDR乳腺癌(BC)治疗的靶向shRNA。首先合成DNA-PLA结合物,然后自组装成两亲性DNA-PLA胶束。接下来,使用结合的DNA作为启动子,通过RCT在DNA-PLA胶束上合成poly-shRNA,产生PLA @ poly-shRNA微型花。最后,使用生物相容的多功能聚乙二醇接枝多肽(PPT- g- PEG)将微花静电凝结成纳米颗粒。这些PLA @ poly-shRNA @ PPT - g - PEG纳米颗粒可以有效地传递到MDR乳腺癌细胞中并积聚在异种移植肿瘤中,从而导致MDR1沉默,细胞内Dox积累,增强的凋亡和增强的肿瘤治疗功效。总体而言,该纳米医学平台有望用于编码交付抗癌核酸疗法和化学疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号