当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteome-Wide Characterization of Phosphorylation-Induced Conformational Changes in Breast Cancer
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acs.jproteome.7b00795 He Meng 1 , Michael C. Fitzgerald 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acs.jproteome.7b00795 He Meng 1 , Michael C. Fitzgerald 1
Affiliation
|
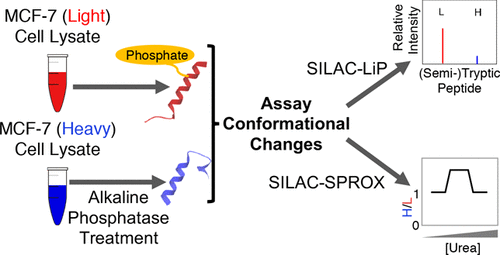
Because of the close link between protein function and protein folding stability, knowledge about phosphorylation-induced protein folding stability changes can lead to a better understanding of the functional effects of protein phosphorylation. Here, the stability of proteins from rates of oxidation (SPROX) and limited proteolysis (LiP) techniques are used to compare the conformational properties of proteins in two MCF-7 cell lysates including one that was and one that was not dephosphorylated with alkaline phosphatase. A total of 168 and 251 protein hits were identified with dephosphorylation-induced stability changes using the SPROX and LiP techniques, respectively. Many protein hits are previously known to be differentially phosphorylated or differentially stabilized in different human breast cancer subtypes, suggesting that the phosphorylation-induced stability changes detected in this work are disease related. The SPROX hits were enriched in proteins with aminoacyl-tRNA ligase activity. These enriched protein hits included many aminoacyl-tRNA synthetases (aaRSs), which are known from previous studies to have their catalytic activity modulated by phosphorylation. The SPROX results revealed that the magnitudes of the destabilizing effects of dephoshporylation on the different aaRSs were directly correlated with their previously reported aminoacylation activity change upon dephosphorylation. This substantiates the close link between protein folding and function.
中文翻译:

磷酸化诱导的乳腺癌构象变化的蛋白质组学表征。
由于蛋白质功能和蛋白质折叠稳定性之间的紧密联系,因此有关磷酸化诱导的蛋白质折叠稳定性变化的知识可以使人们更好地理解蛋白质磷酸化的功能作用。此处,利用氧化速率(SPROX)和有限蛋白水解(LiP)技术的蛋白质稳定性来比较两种MCF-7细胞裂解液中蛋白质的构象特性,其中一种是碱性磷酸酶,另一种是没有被碱性磷酸酶去磷酸化。使用SPROX和LiP技术分别通过脱磷酸化诱导的稳定性变化鉴定出总共168个和251个蛋白质命中。先前已知许多蛋白质命中在不同的人类乳腺癌亚型中差异磷酸化或差异稳定,提示这项工作中检测到的磷酸化诱导的稳定性变化与疾病有关。SPROX命中富含具有氨酰基-tRNA连接酶活性的蛋白质。这些丰富的蛋白质命中物包括许多氨酰基-tRNA合成酶(aaRSs),从以前的研究中可以知道它们的催化活性受磷酸化作用的调节。SPROX结果表明,去磷酸化对不同aaRS的去稳定作用的强度与其先前报道的去磷酸化后的氨酰化活性变化直接相关。这证实了蛋白质折叠和功能之间的紧密联系。这些丰富的蛋白质命中物包括许多氨酰基-tRNA合成酶(aaRSs),从以前的研究中可以知道它们的催化活性受磷酸化作用的调节。SPROX结果表明,去磷酸化对不同aaRS的去稳定作用的强度与其先前报道的去磷酸化后的氨酰化活性变化直接相关。这证实了蛋白质折叠和功能之间的紧密联系。这些丰富的蛋白质命中物包括许多氨酰基-tRNA合成酶(aaRSs),从以前的研究中可以知道它们的催化活性受磷酸化作用的调节。SPROX结果表明,去磷酸化对不同aaRS的去稳定作用的强度与其先前报道的去磷酸化后的氨酰化活性变化直接相关。这证实了蛋白质折叠和功能之间的紧密联系。
更新日期:2018-01-31
中文翻译:

磷酸化诱导的乳腺癌构象变化的蛋白质组学表征。
由于蛋白质功能和蛋白质折叠稳定性之间的紧密联系,因此有关磷酸化诱导的蛋白质折叠稳定性变化的知识可以使人们更好地理解蛋白质磷酸化的功能作用。此处,利用氧化速率(SPROX)和有限蛋白水解(LiP)技术的蛋白质稳定性来比较两种MCF-7细胞裂解液中蛋白质的构象特性,其中一种是碱性磷酸酶,另一种是没有被碱性磷酸酶去磷酸化。使用SPROX和LiP技术分别通过脱磷酸化诱导的稳定性变化鉴定出总共168个和251个蛋白质命中。先前已知许多蛋白质命中在不同的人类乳腺癌亚型中差异磷酸化或差异稳定,提示这项工作中检测到的磷酸化诱导的稳定性变化与疾病有关。SPROX命中富含具有氨酰基-tRNA连接酶活性的蛋白质。这些丰富的蛋白质命中物包括许多氨酰基-tRNA合成酶(aaRSs),从以前的研究中可以知道它们的催化活性受磷酸化作用的调节。SPROX结果表明,去磷酸化对不同aaRS的去稳定作用的强度与其先前报道的去磷酸化后的氨酰化活性变化直接相关。这证实了蛋白质折叠和功能之间的紧密联系。这些丰富的蛋白质命中物包括许多氨酰基-tRNA合成酶(aaRSs),从以前的研究中可以知道它们的催化活性受磷酸化作用的调节。SPROX结果表明,去磷酸化对不同aaRS的去稳定作用的强度与其先前报道的去磷酸化后的氨酰化活性变化直接相关。这证实了蛋白质折叠和功能之间的紧密联系。这些丰富的蛋白质命中物包括许多氨酰基-tRNA合成酶(aaRSs),从以前的研究中可以知道它们的催化活性受磷酸化作用的调节。SPROX结果表明,去磷酸化对不同aaRS的去稳定作用的强度与其先前报道的去磷酸化后的氨酰化活性变化直接相关。这证实了蛋白质折叠和功能之间的紧密联系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号