Molecular Cell ( IF 14.5 ) Pub Date : 2018-01-11 , DOI: 10.1016/j.molcel.2017.12.011 Shinichi Machida , Yoshimasa Takizawa , Masakazu Ishimaru , Yukihiko Sugita , Satoshi Sekine , Jun-ichi Nakayama , Matthias Wolf , Hitoshi Kurumizaka
|
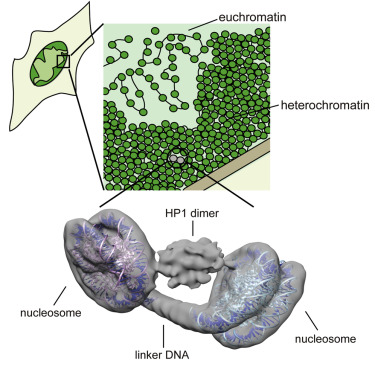
Heterochromatin plays important roles in transcriptional silencing and genome maintenance by the formation of condensed chromatin structures, which determine the epigenetic status of eukaryotic cells. The trimethylation of histone H3 lysine 9 (H3K9me3), a target of heterochromatin protein 1 (HP1), is a hallmark of heterochromatin formation. However, the mechanism by which HP1 folds chromatin-containing H3K9me3 into a higher-order structure has not been elucidated. Here we report the three-dimensional structure of the H3K9me3-containing dinucleosomes complexed with human HP1α, HP1β, and HP1γ, determined by cryogenic electron microscopy with a Volta phase plate. In the structures, two H3K9me3 nucleosomes are bridged by a symmetric HP1 dimer. Surprisingly, the linker DNA between the nucleosomes does not directly interact with HP1, thus allowing nucleosome remodeling by the ATP-utilizing chromatin assembly and remodeling factor (ACF). The structure depicts the fundamental architecture of heterochromatin.
中文翻译:

人类HP1形成异染色质的结构基础
异染色质通过浓缩染色质结构的形成在转录沉默和基因组维持中起重要作用,这决定了真核细胞的表观遗传状态。组蛋白H3赖氨酸9(H3K9me3)的三甲基化是异染色质蛋白1(HP1)的目标,是异染色质形成的标志。但是,尚未阐明HP1将含染色质的H3K9me3折叠成更高阶结构的机制。在这里我们报告与人类HP1α,HP1β和HP1γ复杂的含H3K9me3的二核小体的三维结构,通过具有Volta相板的低温电子显微镜确定。在结构中,两个H3K9me3核小体被对称的HP1二聚体桥接。令人惊讶的是,核小体之间的连接子DNA不直接与HP1相互作用,因此,可以通过利用ATP的染色质装配和重塑因子(ACF)进行核小体重塑。该结构描述了异染色质的基本结构。










































 京公网安备 11010802027423号
京公网安备 11010802027423号