当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unconventional fragment usage enables a concise total synthesis of (−)-callyspongiolide
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b13591 Francesco Manoni 1 , Corentin Rumo 1 , Liubo Li 1 , Patrick G. Harran 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b13591 Francesco Manoni 1 , Corentin Rumo 1 , Liubo Li 1 , Patrick G. Harran 1
Affiliation
|
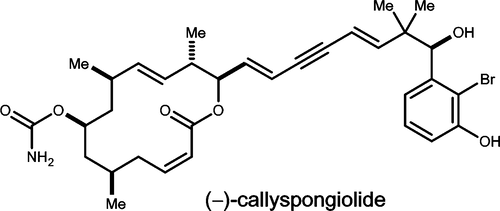
An asymmetric synthesis of (-)-callyspongiolide is described. The route builds the macrolide domain atypically from a disaccharide and a monoterpene without passing through a seco-acid. Chiral iridium catalysis selectively joins fragments. Subsequent degradation of an imbedded butyrolactone via perhemiketal fragmentation affords a stereo- and regio-defined homoallylic alcohol that is engaged directly in a carbonylative macrolactonization. Further elaboration of the polyunsaturated appendage provides the natural product in a particularly direct and flexible manner.
中文翻译:

非常规片段的使用使 (-)-callyspongiolide 的简明全合成成为可能
描述了 (-)-callyspongiolide 的不对称合成。该路线非典型地从二糖和单萜构建大环内酯结构域,而无需通过节酸。手性铱催化选择性地连接片段。随后通过全缩酮裂解嵌入的丁内酯降解提供立体和区域定义的高烯丙醇,其直接参与羰基化大环内酯化。多不饱和附属物的进一步加工以特别直接和灵活的方式提供了天然产品。
更新日期:2018-01-19
中文翻译:

非常规片段的使用使 (-)-callyspongiolide 的简明全合成成为可能
描述了 (-)-callyspongiolide 的不对称合成。该路线非典型地从二糖和单萜构建大环内酯结构域,而无需通过节酸。手性铱催化选择性地连接片段。随后通过全缩酮裂解嵌入的丁内酯降解提供立体和区域定义的高烯丙醇,其直接参与羰基化大环内酯化。多不饱和附属物的进一步加工以特别直接和灵活的方式提供了天然产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号