Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.tet.2018.01.021 Christopher F. Bender , Christopher L. Paradise , Vincent M. Lynch , Francis K. Yoshimoto , Jef K. De Brabander
|
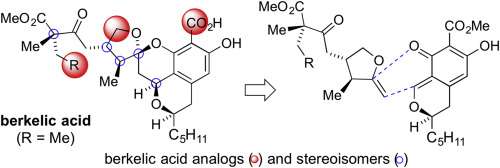
We describe a complete account of our total synthesis and biological evaluation of (−)-berkelic acid and analogs. We delineate a synthetic strategy inspired by a potentially biomimetic union between the natural products spicifernin and pulvilloric acid. After defining optimal parameters, we executed a one-pot silver-mediated in situ dehydration of an isochroman lactol to methyl pulvillorate, the cycloisomerization of a spicifernin-like alkynol to the corresponding exocyclic enol ether, and a subsequent cycloaddition to deliver the tetracyclic core of berkelic acid. Our studies confirm that the original assigned berkelic acid structure is not stable and equilibrates into a mixture of 4 diastereomers, fully characterized by X-ray crystallography. In addition to berkelic acid, C22-epi-berkelic acid, and nor-berkelic acids, we synthesized C26-oxoberkelic acid analogs that were evaluated against human cancer cell lines. In contrast to data reported for natural berkelic acid, our synthetic material and analogs were found to be devoid of activity.
中文翻译:

(-)-伯酸和类似物的生物合成启发性合成
我们描述了我们对(-)-伯酸和类似物的总合成和生物学评估的完整说明。我们描述了一种合成策略,该策略受到天然产物spicifernin和pulvilloric酸之间潜在的仿生结合的启发。定义最佳参数后,我们进行了一锅银介导的异色满乳糖醇的原位脱水,制成了pulvillorate甲酯,将spicifernin样炔醇进行了环异构化为相应的环外烯醇醚,随后进行了环加成反应,从而得到了铍酸。我们的研究证实,最初分配的铍酸结构不稳定,并且会平衡为4个非对映异构体的混合物,并通过X射线晶体学对其进行了充分表征。除了铍酸,C22-表-伯酸和也不-berkelic酸,我们合成了被针对人癌细胞系评价C26-oxoberkelic酸类似物。与报道的天然铍酸的数据相反,我们的合成材料和类似物被发现没有活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号