PLOS ONE ( IF 2.9 ) Pub Date : 2018-01-12 , DOI: 10.1371/journal.pone.0191069 N Chantal Peltenburg 1, 2 , Jörgen Bierau 3 , Jaap A Bakker 4 , Jolanda A Schippers 5 , Selwyn H Lowe 6, 7 , Aimée D C Paulussen 3 , Bianca J C van den Bosch 3 , Mathie P G Leers 8 , Bettina E Hansen 9 , Annelies Verbon 1, 2
|
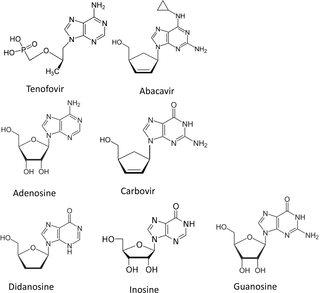
The purine analogues tenofovir and abacavir are precursors of potential substrates for the enzyme Inosine 5’-triphosphate pyrophosphohydrolase (ITPase). Here, we investigated the association of ITPase activity and ITPA genotype with the occurrence of adverse events (AEs) during combination antiretroviral therapy (cART) for human immunodeficiency virus (HIV) infection. In 393 adult HIV-seropositive patients, AEs were defined as events that led to stop of cART regimen. ITPase activity ≥4 mmol IMP/mmol Hb/hour was considered as normal. ITPA genotype was determined by testing two ITPA polymorphisms: c.94C>A (p.Pro32Thr, rs1127354) and c.124+21A>C (rs7270101). Logistic regression analysis determined odds ratios for developing AEs. In tenofovir-containing regimens decreased ITPase activity was associated with less AEs (p = 0.01) and longer regimen duration (p = 0.001). In contrast, in abacavir-containing regimens decreased ITPase activity was associated with more AEs (crude p = 0.02) and increased switching of medication due to AEs (p = 0.03). ITPA genotype wt/wt was significantly associated with an increase in the occurrence of AEs in tenofovir-containing regimens. Decreased ITPase activity seems to be protective against occurrence of AEs in tenofovir-containing cART, while it is associated with an increase in AEs in abacavir-containing regimens.
中文翻译:

红细胞肌苷三磷酸酶活性:HIV联合抗逆转录病毒治疗期间不良事件的潜在生物标志物
嘌呤类似物替诺福韦和阿巴卡韦是肌苷 5'-三磷酸焦磷酸水解酶 (ITPase) 潜在底物的前体。在这里,我们研究了 ITPase 活性和ITPA基因型与人类免疫缺陷病毒 (HIV) 感染联合抗逆转录病毒治疗 (cART) 期间不良事件 (AE) 发生的关系。在 393 名成年 HIV 血清阳性患者中,AE 被定义为导致 cART 治疗方案停止的事件。 ITPase 活性≥4 mmol IMP/mmol Hb/小时被认为是正常的。通过测试两个ITPA多态性来确定ITPA基因型:c.94C>A(p.Pro32Thr,rs1127354)和c.124+21A>C(rs7270101)。逻辑回归分析确定了发生 AE 的优势比。在含替诺福韦的治疗方案中,ITPase 活性降低与 AE 较少 (p = 0.01) 和治疗持续时间较长 (p = 0.001) 相关。相比之下,在含阿巴卡韦的方案中,ITPase 活性降低与更多 AE 相关(粗略 p = 0.02),并且由于 AE 导致药物转换增加(p = 0.03)。 ITPA基因型 wt/wt 与含替诺福韦治疗方案中 AE 发生率的增加显着相关。 ITPase 活性的降低似乎可以防止含替诺福韦的 cART 中 AE 的发生,而它与含阿巴卡韦的方案中 AE 的增加有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号