当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Reaction Mechanism of the MoS2 Electrode in a Lithium-Ion Cell Revealed by in Situ and Operando X-ray Absorption Spectroscopy
Nano Letters ( IF 9.6 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.nanolett.7b05246 Liang Zhang , Dan Sun , Jun Kang , Jun Feng , Hans A. Bechtel , Lin-Wang Wang , Elton J. Cairns 1 , Jinghua Guo 2
Nano Letters ( IF 9.6 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.nanolett.7b05246 Liang Zhang , Dan Sun , Jun Kang , Jun Feng , Hans A. Bechtel , Lin-Wang Wang , Elton J. Cairns 1 , Jinghua Guo 2
Affiliation
|
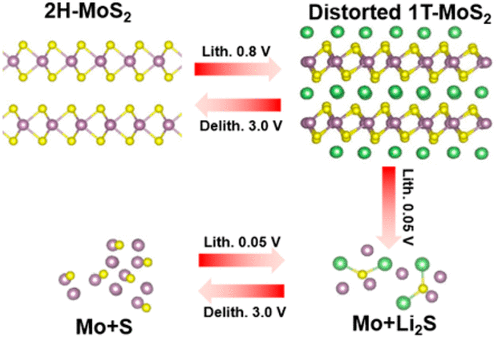
As a typical transition metal dichalcogenide, MoS2 offers numerous advantages for nanoelectronics and electrochemical energy storage due to its unique layered structure and tunable electronic properties. When used as the anode in lithium-ion cells, MoS2 undergoes intercalation and conversion reactions in sequence upon lithiation, and the reversibility of the conversion reaction is an important but still controversial topic. Here, we clarify unambiguously that the conversion reaction of MoS2 is not reversible, and the formed Li2S is converted to sulfur in the first charge process. Li2S/sulfur becomes the main redox couple in the subsequent cycles and the main contributor to the reversible capacity. In addition, due to the insulating nature of both Li2S and sulfur, a strong relaxation effect is observed during the cycling process. This study clearly reveals the electrochemical lithiation–delithiation mechanism of MoS2, which can facilitate further developments of high-performance MoS2-based electrodes.
中文翻译:

原位和操作X射线吸收光谱揭示锂离子电池中MoS 2电极的电化学反应机理
作为典型的过渡金属二卤化二硫化钼,由于其独特的分层结构和可调节的电子特性,MoS 2在纳米电子学和电化学储能方面具有众多优势。当用作锂离子电池的阳极时,MoS 2会在锂化过程中依次经历嵌入和转化反应,转化反应的可逆性是一个重要但仍存在争议的话题。在此,我们明确地阐明,MoS 2的转化反应是不可逆的,并且在第一充电过程中将形成的Li 2 S转化为硫。李2在随后的循环中,S /硫成为主要的氧化还原对,并且是可逆容量的主要贡献者。另外,由于Li 2 S和硫的绝缘性质,在循环过程中观察到强烈的松弛效果。这项研究清楚地揭示了MoS 2的电化学锂化-去锂化机理,可以促进高性能基于MoS 2的电极的进一步发展。
更新日期:2018-01-18
中文翻译:

原位和操作X射线吸收光谱揭示锂离子电池中MoS 2电极的电化学反应机理
作为典型的过渡金属二卤化二硫化钼,由于其独特的分层结构和可调节的电子特性,MoS 2在纳米电子学和电化学储能方面具有众多优势。当用作锂离子电池的阳极时,MoS 2会在锂化过程中依次经历嵌入和转化反应,转化反应的可逆性是一个重要但仍存在争议的话题。在此,我们明确地阐明,MoS 2的转化反应是不可逆的,并且在第一充电过程中将形成的Li 2 S转化为硫。李2在随后的循环中,S /硫成为主要的氧化还原对,并且是可逆容量的主要贡献者。另外,由于Li 2 S和硫的绝缘性质,在循环过程中观察到强烈的松弛效果。这项研究清楚地揭示了MoS 2的电化学锂化-去锂化机理,可以促进高性能基于MoS 2的电极的进一步发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号